Answer:
The transition elements show a
large number of oxidation states. The various oxidation states are related to
the electronic configuration of their atoms. The various oxidation states of a
transition metal is due to the involvement of \[(n-1)d\] and outer ns
electrons in bonding.
The lower oxidation state is
generally shown when ns electrons participate and higher oxidation states are
exhibited when ns and \[(n-1)d\] electrons take part in bonding. For example,
manganese, electronic configuration
\[\left( n-1
\right){{d}^{5}}n{{s}^{2}}\], can show +2, +3, +4, +6 and +7 oxidation states.
In the first five elements of
the first transition series, i.e., upto manganese, the maximum oxidation state
is equal to the sum of 4s and 3d electrons. For remaining five elements, the
maximum state is not related to their electronic configurations.
The non-transition elements
mainly the p-block elements can show a number of oxidation states from +n to \[(n-8)\]
where n is the number of electrons present in the outermost shell. For
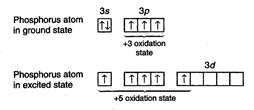
example, phosphorus can show -3, +3 and +5 oxidation states while sulphur can
show -2, +2, +4 and +6 oxidation states. Iodine can show -1, +1, +3, +5 and +7 oxidation
states. Lower oxidation states are ionic as the atom accepts the electron or
electrons to achieve stable configuration while higher oxidation states are
achieved by unpairing the paired orbitals and shifting the electrons to vacant
d-orbitals.
You need to login to perform this action.
You will be redirected in
3 sec
