Answer:
(c,
d)
In\[CO_{3}^{2-}\],
the hybridisation of carbon atom is \[s{{p}^{2}}\] and not\[s{{p}^{3}}\]. Its
resonance structure is :
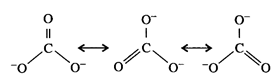 i.e.,
the resonance structure has two C - O bonds and one C = O bond.
Formal
charge on double bond oxygen = 0
Formal
charge on single bonded oxygen = -1
Average
formal charge on oxygen =
\[\frac{0-1-1}{3}=-\frac{2}{3}\]
= -
0.67
All C - O
bond lengths are same.
i.e.,
the resonance structure has two C - O bonds and one C = O bond.
Formal
charge on double bond oxygen = 0
Formal
charge on single bonded oxygen = -1
Average
formal charge on oxygen =
\[\frac{0-1-1}{3}=-\frac{2}{3}\]
= -
0.67
All C - O
bond lengths are same.
You need to login to perform this action.
You will be redirected in
3 sec
