Answer:
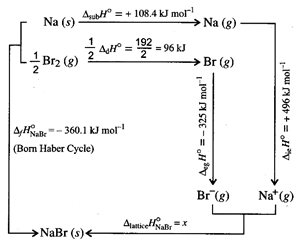 \[{{\Delta
}_{f}}H_{NaBr}^{{}^\circ }={{\Delta }_{sub}}{{H}^{{}^\circ
}}+\frac{1}{2}{{\Delta }_{d}}{{H}^{{}^\circ }}+{{\Delta }_{ie}}{{H}^{{}^\circ
}}+{{\Delta }_{eg}}{{H}^{{}^\circ }}+{{\Delta }_{lattice}}H{}^\circ \]
\[-360.1=+108.4+96+496-325+{{\Delta
}_{lattice}}{{H}^{{}^\circ }}\]
\[{{\Delta
}_{lattice}}{{H}^{{}^\circ }}=-360.1-108.4-96-496+325\]
\[=-735.5kJ\,mo{{l}^{-1}}\]
\[{{\Delta
}_{f}}H_{NaBr}^{{}^\circ }={{\Delta }_{sub}}{{H}^{{}^\circ
}}+\frac{1}{2}{{\Delta }_{d}}{{H}^{{}^\circ }}+{{\Delta }_{ie}}{{H}^{{}^\circ
}}+{{\Delta }_{eg}}{{H}^{{}^\circ }}+{{\Delta }_{lattice}}H{}^\circ \]
\[-360.1=+108.4+96+496-325+{{\Delta
}_{lattice}}{{H}^{{}^\circ }}\]
\[{{\Delta
}_{lattice}}{{H}^{{}^\circ }}=-360.1-108.4-96-496+325\]
\[=-735.5kJ\,mo{{l}^{-1}}\]
You need to login to perform this action.
You will be redirected in
3 sec
