Answer:
1.\[\mathbf{s}{{\mathbf{p}}^{\mathbf{3}}}\]d-Hybridization
: It involves the mixing of one s-, three p- and one d-orbitals resulting
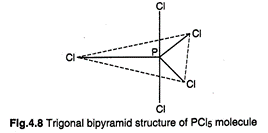
in the formation of trigonal bipyramidal arrangement. In this arrangement, the three
hybrid orbitals forming a plane, are directed towards the corners of an
equilateral triangle while the other two hybrid orbitals are perpendicular to
the plane of a triangle lying above and below it. \[PC{{l}_{5}}\] is an example
of this type of hybridization.
\[\mathbf{PC}{{\mathbf{l}}_{\mathbf{5}}}\]
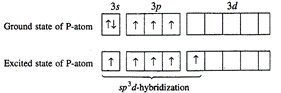
molecule: P-atom has configuration \[1{{s}^{2}},\text{ }2{{s}^{2}},\text{
}2{{p}^{6}},\]\[3{{s}^{2}}3p_{x}^{1},3p_{y}^{1},3p_{z}^{1}\]In ground state, it
can form three covalent bonds as three unpaired orbitals are present in the
valency shell. To get pentavalency, 3s-orbital is unpaired and the electron is
shifted to 3d-orbital. Now in the excited state the five orbitals involving one
s-, one d- and three p-orbitals undergo hybridization giving birth to five hybrid
orbitals which overlap with five chlorine atoms forming five sigma bonds. Out
of five \[\sigma \]-bonds, three bonds which are located at\[120{}^\circ \]
angle are equitorial and the remaining two are axial.
Axial
bond length is greater.
 Each
P-Cl axial bond length =219 pm and each P-Cl equatorial bond length = 204 pm.
Axial bonds are thus slightly weaker than equatorial bonds.
\[P{{F}_{5}}\]
molecule has similar hybridization and geometry.
2.\[\mathbf{s}{{\mathbf{p}}^{\mathbf{3}}}{{\mathbf{d}}^{\mathbf{2}}}\]-Hybridization:
It involves the mixing of one\[s\]-, three p- and two d-orbitals resulting in
the formation of octahedral arrangement, \[S{{F}_{6}}\] is an example of this
type of hybridization.
In\[S{{F}_{6}}\],
the central sulphur atom has the ground state configuration \[3{{s}^{2}}3p_{x}^{2}3p_{y}^{1}3p_{z}^{1}\]to
account for hexavalency, the paired 3s and paired \[3{{p}_{x}}\] are unpaired
and electrons are shifted to d-orbitals. These six orbitals undergo
hybridization giving six hybrid orbitals directed towards the comers of a
regular octahedron. Each of these hybrid orbitals overlaps with 2p- orbital of
fluorine to form S?F sigma bond. Thus, \[S{{F}_{6}}\] molecule has octahedral structure.
Each
P-Cl axial bond length =219 pm and each P-Cl equatorial bond length = 204 pm.
Axial bonds are thus slightly weaker than equatorial bonds.
\[P{{F}_{5}}\]
molecule has similar hybridization and geometry.
2.\[\mathbf{s}{{\mathbf{p}}^{\mathbf{3}}}{{\mathbf{d}}^{\mathbf{2}}}\]-Hybridization:
It involves the mixing of one\[s\]-, three p- and two d-orbitals resulting in
the formation of octahedral arrangement, \[S{{F}_{6}}\] is an example of this
type of hybridization.
In\[S{{F}_{6}}\],
the central sulphur atom has the ground state configuration \[3{{s}^{2}}3p_{x}^{2}3p_{y}^{1}3p_{z}^{1}\]to
account for hexavalency, the paired 3s and paired \[3{{p}_{x}}\] are unpaired
and electrons are shifted to d-orbitals. These six orbitals undergo
hybridization giving six hybrid orbitals directed towards the comers of a
regular octahedron. Each of these hybrid orbitals overlaps with 2p- orbital of
fluorine to form S?F sigma bond. Thus, \[S{{F}_{6}}\] molecule has octahedral structure.
![]()
![]() \[S{{F}_{6}}\]
is a symmetrical molecule and therefore, is stable and
\[S{{F}_{6}}\]
is a symmetrical molecule and therefore, is stable and
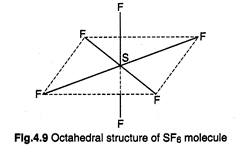 far less reactive. Four S-F bonds are in the same plane at right
angles to one another and are directed towards four corners of a square. The
other two F-atoms lie at right angle above and below the plane.
far less reactive. Four S-F bonds are in the same plane at right
angles to one another and are directed towards four corners of a square. The
other two F-atoms lie at right angle above and below the plane.
You need to login to perform this action.
You will be redirected in
3 sec
