Answer:
As is clear from Fig. 8(NCT).1,
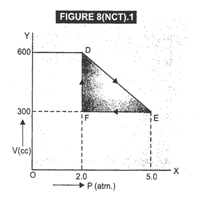 Change in pressure,
Change in pressure,
![]()
![]() Change in volume, dV =EF= 600 -
300 = 300 cc
Change in volume, dV =EF= 600 -
300 = 300 cc
![]() Work
done by the. gas from D to E to F= area of
Work
done by the. gas from D to E to F= area of ![]()
![]()
![]()
You need to login to perform this action.
You will be redirected in
3 sec
