Answer:
\[BC{{l}_{3}}\] exists as
monomer in spite of the fact that it is an electron deficient compound. It
achieves stability by forming \[p\pi -p\pi \] back bonding. Chlorine transfers
two electrons to vacant 2p-orbital of boron.
All the three bond lengths are
same. Thus, the back
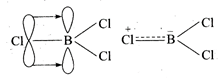 bonding giving double bond
character is delocalized in the molecule.
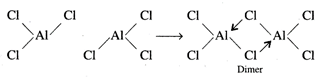
\[AlC{{l}_{3}}\]is also an
electron deficient compound. Since, aluminium has larger size than boron, the
back bonding is not possible in\[AlC{{l}_{3}}\]. Aluminium metal complete its
octet by forming coordinate bond with chlorine atom of the other molecule,\[AlC{{l}_{3}}\].
Thus, coordinate bond forming bridges by chlorine atoms between two Al atoms
make a dimer molecule.
bonding giving double bond
character is delocalized in the molecule.
\[AlC{{l}_{3}}\]is also an
electron deficient compound. Since, aluminium has larger size than boron, the
back bonding is not possible in\[AlC{{l}_{3}}\]. Aluminium metal complete its
octet by forming coordinate bond with chlorine atom of the other molecule,\[AlC{{l}_{3}}\].
Thus, coordinate bond forming bridges by chlorine atoms between two Al atoms
make a dimer molecule.
You need to login to perform this action.
You will be redirected in
3 sec
