Answer:
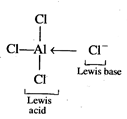
(i) \[AlC{{l}_{3}}\]is electron
deficient molecule hence it acts as Lewis acid.
 (ii) In\[B{{F}_{3}}\], boron has
a vacant 2p-orbital and each fluorine has fully filled unutilized -orbitals.
Fluorine transfer two electrons to vacant 2p-orbital of boron forming \[p\pi
-p\pi \]bond. This is known as back bonding. This bond reduces the electron
deficiency of boron atom hence its Lewis acid character decreases.
This tendency of back bonding is
maximum in \[B{{F}_{3}}\] and of decreases from \[B{{F}_{3}}\] to \[BC{{l}_{3}}\]
on account of increase in size of chlorine atom.
(iii) In \[Pb{{O}_{2}}\] and\[Sn{{O}_{2}}\],
the metals are present in (+4) state. In lead \[P{{b}^{2+}}\] state is more
stable than \[P{{b}^{4+}}\]state due to inert pair effect. Thus, \[P{{b}^{4+}}\]
tries to attain more stable state i.e., \[P{{b}^{2+}}\], by acting as an
oxidising agent.
\[P{{b}^{4+}}+2{{e}^{-}}\xrightarrow{{}}P{{b}^{2+}}\]
In tin, \[S{{n}^{2+}}\] state is
less stable than\[S{{n}^{4+}}\]. Thus,\[S{{n}^{2+}}\]tries to attain more stable
configuration i.e., \[S{{n}^{4+}}\]state by acting as a reducing agent.
\[S{{n}^{2+}}\xrightarrow{{}}S{{n}^{4+}}+2{{e}^{-}}\]
(iv) The stability of +1
oxidation state is maximum in thallium amongst group 13 elements as the inert
pair effect is maximum in thallium due to poor shielding effect of d-and f-electrons
present in the inner energy shells on\[n{{s}^{2}}\]-electrons.
(ii) In\[B{{F}_{3}}\], boron has
a vacant 2p-orbital and each fluorine has fully filled unutilized -orbitals.
Fluorine transfer two electrons to vacant 2p-orbital of boron forming \[p\pi
-p\pi \]bond. This is known as back bonding. This bond reduces the electron
deficiency of boron atom hence its Lewis acid character decreases.
This tendency of back bonding is
maximum in \[B{{F}_{3}}\] and of decreases from \[B{{F}_{3}}\] to \[BC{{l}_{3}}\]
on account of increase in size of chlorine atom.
(iii) In \[Pb{{O}_{2}}\] and\[Sn{{O}_{2}}\],
the metals are present in (+4) state. In lead \[P{{b}^{2+}}\] state is more
stable than \[P{{b}^{4+}}\]state due to inert pair effect. Thus, \[P{{b}^{4+}}\]
tries to attain more stable state i.e., \[P{{b}^{2+}}\], by acting as an
oxidising agent.
\[P{{b}^{4+}}+2{{e}^{-}}\xrightarrow{{}}P{{b}^{2+}}\]
In tin, \[S{{n}^{2+}}\] state is
less stable than\[S{{n}^{4+}}\]. Thus,\[S{{n}^{2+}}\]tries to attain more stable
configuration i.e., \[S{{n}^{4+}}\]state by acting as a reducing agent.
\[S{{n}^{2+}}\xrightarrow{{}}S{{n}^{4+}}+2{{e}^{-}}\]
(iv) The stability of +1
oxidation state is maximum in thallium amongst group 13 elements as the inert
pair effect is maximum in thallium due to poor shielding effect of d-and f-electrons
present in the inner energy shells on\[n{{s}^{2}}\]-electrons.
You need to login to perform this action.
You will be redirected in
3 sec
