Answer:
The carbon, the first member of
group 14 possesses a pronounced ability to form stable \[p\pi -p\pi \] multiple
bonds with itself and with other first row elements such as nitrogen and
oxygen. In\[C{{O}_{2}}\], both the oxygen atoms are linked with carbon atom by
double bonds.
![]() However, silicon shows its
reluctance in forming \[p\pi -p\pi \]multiple bonding due to large atomic size.
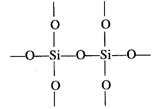
Thus, in\[Si{{O}_{2}}\],oxygen atoms are linked to silicon atom by single
covalent bonds giving three dimensional network.
However, silicon shows its
reluctance in forming \[p\pi -p\pi \]multiple bonding due to large atomic size.
Thus, in\[Si{{O}_{2}}\],oxygen atoms are linked to silicon atom by single
covalent bonds giving three dimensional network.
You need to login to perform this action.
You will be redirected in
3 sec
