Answer:
(a) Because of its small size
and good \[\pi \]-overlap with other small atoms, carbon forms strong double
bonds with two oxygen atoms to give discrete \[C{{O}_{2}}\] molecules.
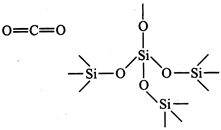
Silicon atom, on account of
large size, does not have good\[\pi \]-overlap with other atoms. It uses its
four valence electrons to form four single bonds directed towards the four
apices of a tetrahedron (\[s{{p}^{3}}\]-hybridization). Each oxygen is linked
with two silicon atoms, i.e., a giant three dimensional structure comes into
existence which is very stable. Thus, \[C{{O}_{2}}\] is a gas and \[Si{{O}_{2}}\]
is a solid.
 (b) Silicon has 3d-orbitals in
the valence shell and thus expands its octet giving \[s{{p}^{3}}{{d}^{2}}\]-hybridization
while d-orbitals are not present in the valence shell of carbon. It can undergo
sp3 -hybridization only. Thus, carbon is unable to form \[CF_{6}^{2-}\] anion.
(b) Silicon has 3d-orbitals in
the valence shell and thus expands its octet giving \[s{{p}^{3}}{{d}^{2}}\]-hybridization
while d-orbitals are not present in the valence shell of carbon. It can undergo
sp3 -hybridization only. Thus, carbon is unable to form \[CF_{6}^{2-}\] anion.
You need to login to perform this action.
You will be redirected in
3 sec
