Answer:
(a)\[CC{{l}_{4}}\]is
immiscible in water as it is a covalent compound and is not hydrolysed by water
because carbon does not have d-orbitals and hence cannot expand its
coordination number beyond 4.
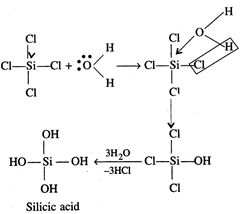
However, silicon can expand its
coordination number beyond 4 due to availability of d-orbitals.
\[CC{{l}_{4}}+{{H}_{2}}O\xrightarrow{{}}No\,reaction\]
\[SiC{{l}_{4}}+4{{H}_{2}}O\xrightarrow{{}}Si{{(OH)}_{4}}+4HCl\]
 (b) The property of catenation depends
upon the strength of element-element bond. Since, the bond energy of C-C bond
is very large\[(348kJ\,mo{{l}^{-1}})\], carbon has strong tendency of
catenation, while the bond energy of Si-Si bond is comparatively less \[(297\,kJmo{{l}^{-1}})\]and
it has lesser property of catenation.
(b) The property of catenation depends
upon the strength of element-element bond. Since, the bond energy of C-C bond
is very large\[(348kJ\,mo{{l}^{-1}})\], carbon has strong tendency of
catenation, while the bond energy of Si-Si bond is comparatively less \[(297\,kJmo{{l}^{-1}})\]and
it has lesser property of catenation.
You need to login to perform this action.
You will be redirected in
3 sec
