Answer:
(c) \[{{H}_{3}}B{{O}_{3}}\]or\[{{H}_{3}}B{{O}_{3}}\]
in aqueous solution coordinates with a water molecule to form a hydrated
species.
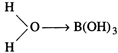 \[{{B}^{3+}}\]ion pulls the \[\sigma
\]-electron charge of the coordinated O-atom towards itself. The coordinated
oxygen, in turn, pulls the \[\sigma \]-electron charge of the O-H bond of the attached
water molecule. This facilitates the removal of \[{{H}^{+}}\] ion from the O-H
bond. Thus, the aqueous solution of\[{{H}_{3}}B{{O}_{3}}\] acts as a weak
acid.
\[{{B}^{3+}}\]ion pulls the \[\sigma
\]-electron charge of the coordinated O-atom towards itself. The coordinated
oxygen, in turn, pulls the \[\sigma \]-electron charge of the O-H bond of the attached
water molecule. This facilitates the removal of \[{{H}^{+}}\] ion from the O-H
bond. Thus, the aqueous solution of\[{{H}_{3}}B{{O}_{3}}\] acts as a weak
acid.
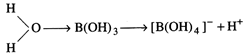 or\[B{{(OH)}_{3}}\] accepts \[O{{H}^{-}}\]
from water and releases \[{{H}^{+}}\] and it acts as a weak acid.
or\[B{{(OH)}_{3}}\] accepts \[O{{H}^{-}}\]
from water and releases \[{{H}^{+}}\] and it acts as a weak acid.
You need to login to perform this action.
You will be redirected in
3 sec
