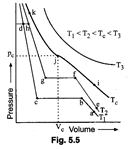
 Answer the following questions
based on this figure.
(i) In which state will \[C{{O}_{2}}\]
exist between the points a and bat temperature\[{{T}_{1}}\]?
(ii) At what point will \[C{{O}_{2}}\]start
liquefying when temperature is \[{{T}_{1}}\]?
(iii) At what point will \[C{{O}_{2}}\]
be completely liquefied when temperature is\[{{T}_{2}}\]?
(iv) Will condensation take
place when the temperature is\[{{T}_{3}}\]?
(v) What portion of the isotherm
at represent liquid and gaseous \[C{{O}_{2}}\] at equilibrium?
Answer the following questions
based on this figure.
(i) In which state will \[C{{O}_{2}}\]
exist between the points a and bat temperature\[{{T}_{1}}\]?
(ii) At what point will \[C{{O}_{2}}\]start
liquefying when temperature is \[{{T}_{1}}\]?
(iii) At what point will \[C{{O}_{2}}\]
be completely liquefied when temperature is\[{{T}_{2}}\]?
(iv) Will condensation take
place when the temperature is\[{{T}_{3}}\]?
(v) What portion of the isotherm
at represent liquid and gaseous \[C{{O}_{2}}\] at equilibrium?
Answer:
(i) \[C{{O}_{2}}\] will exist in
gaseous state between the points 'a' and ?b?
(ii) At temperature \[{{T}_{1}},C{{O}_{2}}\]
will start liquefaction at point ?b?
(iii) At temperature \[{{T}_{2}},C{{O}_{2}}\]
will start liquefaction at \['f'\] and will completely liquefy at ?g?.
(iv) No, because a gas cannot be
liquefied above the critical temperature. \[({{T}_{3}}>{{T}_{c}})\]
(v) At temperature\[{{T}_{2}}\],
liquid and gas will exist in equilibrium between the points 'b? and ?c?
You need to login to perform this action.
You will be redirected in
3 sec
