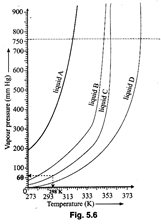
Answer:
(i) The temperature at which the
vapour pressure of a liquid becomes equal to atmospheric pressure (760 mm Hg)
is called its boiling point.
Then, boiling point of liquid
(A) and (B) will be respectively 315 K and 345 K.
(ii) Liquid will not boiled in
closed vessel.
(iii) At 313 K, vapour pressure
of liquid (D) will be atmospheric pressure (60 mm Hg) hence it will boil at 313
K.
(iv) On high altitude, the
atmospheric pressure is low hence water boils at low temperature on hills. We
know that, a liquid boils when its vapour pressure becomes equal to the atmospheric
pressure. Pressure cooker increases the boiling point of water and its heat
content hence food is cooked early.
You need to login to perform this action.
You will be redirected in
3 sec
