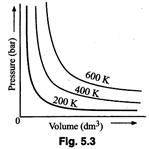
 On the basis of this graph
answer the following questions :
(i) How will the volume of a gas
change if its pressure is increased at constant temperature?
(ii) At a constant pressure, how
will the volume of a gas change if the temperature is increased from 200 K to 400
K?
On the basis of this graph
answer the following questions :
(i) How will the volume of a gas
change if its pressure is increased at constant temperature?
(ii) At a constant pressure, how
will the volume of a gas change if the temperature is increased from 200 K to 400
K?
Answer:
(i) Volume of a gas will
decrease if the pressure is increase at constant temperature. It is evident
from the graph. The graph is in accordance with Boyle's law.
(ii) On increasing the
temperature, volume of gas will increase at constant pressure.
You need to login to perform this action.
You will be redirected in
3 sec
