Answer:
(a) The given statement is true.
(b)The statement is according to
the law of multiple proportion.
(c) \[{{H}_{2}}O\] \[{{H}_{2}}{{O}_{2}}\]
Mass of W : Mass of 'O' Mass of
W : Mass of 'O'
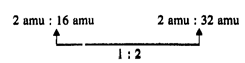 Masses of oxygen which combine
with fixed man of hydrogen are in simple multiple ratio.
Masses of oxygen which combine
with fixed man of hydrogen are in simple multiple ratio.
You need to login to perform this action.
You will be redirected in
3 sec
