Answer:
Steam distillation: This type of distillation is
essentially a co-distillation with water and is carried out when a solid or liquid,
practically insoluble in water, is volatile with steam, possess a vapour
pressure of about 10-15 mm of mercury but the impurities are non-volatile.
The
principle of steam distillation is based on Dalton?s law of partial
pressures. Let \[{{p}_{1}}\] and \[{{p}_{2}}\] be the vapour pressures of
water vapour and the organic liquid at the distillation temperature. The liquid
boils when total pressure is equal to the atmospheric pressure p, i.e.,
\[p={{p}_{1}}+{{p}_{2}}\]
or \[{{p}_{2}}=p-{{p}_{1}}\]
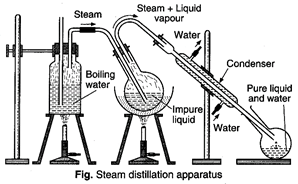 This process is used in the purification
of compounds such as chlorotoluenes, aniline and nitrobenzene. It is also employed
in the isolation of essential oils from flowers.
This process is used in the purification
of compounds such as chlorotoluenes, aniline and nitrobenzene. It is also employed
in the isolation of essential oils from flowers.
You need to login to perform this action.
You will be redirected in
3 sec
