Answer:
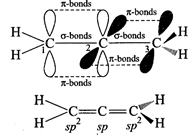 Allene are cumulative
dienes \[{{H}_{2}}\overset{1}{\mathop{C}}\,=\overset{2}{\mathop{C}}\,=\overset{3}{\mathop{C}}\,{{H}_{2}}\]
(Propa-1, 2-diene). In allene \[{{C}_{1}}\]and \[{{C}_{3}}\] are \[s{{p}^{2}}\]?hybridized
and \[{{C}_{2}}\] is \[sp\]hybridized. The two \[\pi \]-bonds are present in
the central carbon, one each from \[{{p}_{y}}\] and \[{{p}_{z}}\] orbitals
thus, overlapping planes in \[{{C}_{1}}\] and \[{{C}_{3}}\] are also different.
Allene are cumulative
dienes \[{{H}_{2}}\overset{1}{\mathop{C}}\,=\overset{2}{\mathop{C}}\,=\overset{3}{\mathop{C}}\,{{H}_{2}}\]
(Propa-1, 2-diene). In allene \[{{C}_{1}}\]and \[{{C}_{3}}\] are \[s{{p}^{2}}\]?hybridized
and \[{{C}_{2}}\] is \[sp\]hybridized. The two \[\pi \]-bonds are present in
the central carbon, one each from \[{{p}_{y}}\] and \[{{p}_{z}}\] orbitals
thus, overlapping planes in \[{{C}_{1}}\] and \[{{C}_{3}}\] are also different.
You need to login to perform this action.
You will be redirected in
3 sec
