
Answer:
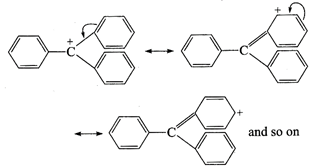 Triphenyl cation is stabilized
by exhaustive resonance, since there are three benzene rings, therefore, in all
nine resonance structures can be given for this ion.
Triphenyl cation is stabilized
by exhaustive resonance, since there are three benzene rings, therefore, in all
nine resonance structures can be given for this ion.
You need to login to perform this action.
You will be redirected in
3 sec
