Answer:
(i) Structure of \[{{H}_{2}}{{O}_{2}}\]
is slightly different in gas phase and solid phase.
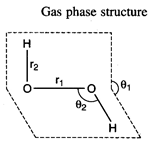 \[{{r}_{1}}=147.5pm,\]\[{{r}_{2}}=95pm\]
\[{{\theta
}_{1}}={{111.5}^{{}^\circ }},\,\,\,\,\,\,{{\theta }_{2}}={{94.8}^{{}^\circ }}\]
\[{{r}_{1}}=147.5pm,\]\[{{r}_{2}}=95pm\]
\[{{\theta
}_{1}}={{111.5}^{{}^\circ }},\,\,\,\,\,\,{{\theta }_{2}}={{94.8}^{{}^\circ }}\]
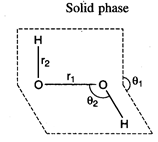 \[{{r}_{1}}=98.5pm,\]\[{{r}_{2}}=145.8pm.\]
\[{{\theta }_{1}}={{90.2}^{{}^\circ
}},\]\[{{\theta }_{2}}={{101.2}^{{}^\circ }}\]
(ii) is a better oxidising agent
than water. \[{{H}_{2}}{{O}_{2}}\]acts as an oxidising agent in acid as well as
in alkaline medium.
\[{{H}_{2}}{{O}_{2}}+2{{H}^{+}}+2e\xrightarrow{Acid}2{{H}_{2}}O\]\[{{E}^{{}^\circ
}}=1.77V\]
\[{{H}_{2}}{{O}_{2}}+O{{H}^{-}}+2e\xrightarrow{Alkaline}3O{{H}^{-}}\]
\[{{E}^{{}^\circ }}=0.88V\]
Oxidation state of oxygen
changes from -1 to -2. Oxidising nature of \[{{H}_{2}}{{O}_{2}}\] can be
interpreted on account of possession of labile oxygen.
\[{{H}_{2}}{{O}_{3}}\to {{H}_{2}}O+O\]
When water acts as an oxidising agent,
it is reduced to\[{{H}_{2}}\].Water reacts with number of active metals whose
electrode potential is less than - 0.83 V.
\[\underset{\text{Reductant}}{\mathop{2Na+}}\,\underset{Oxidant}{\mathop{2{{H}_{2}}O}}\,\to
2NaOH+{{H}_{2}}\]
\[{{r}_{1}}=98.5pm,\]\[{{r}_{2}}=145.8pm.\]
\[{{\theta }_{1}}={{90.2}^{{}^\circ
}},\]\[{{\theta }_{2}}={{101.2}^{{}^\circ }}\]
(ii) is a better oxidising agent
than water. \[{{H}_{2}}{{O}_{2}}\]acts as an oxidising agent in acid as well as
in alkaline medium.
\[{{H}_{2}}{{O}_{2}}+2{{H}^{+}}+2e\xrightarrow{Acid}2{{H}_{2}}O\]\[{{E}^{{}^\circ
}}=1.77V\]
\[{{H}_{2}}{{O}_{2}}+O{{H}^{-}}+2e\xrightarrow{Alkaline}3O{{H}^{-}}\]
\[{{E}^{{}^\circ }}=0.88V\]
Oxidation state of oxygen
changes from -1 to -2. Oxidising nature of \[{{H}_{2}}{{O}_{2}}\] can be
interpreted on account of possession of labile oxygen.
\[{{H}_{2}}{{O}_{3}}\to {{H}_{2}}O+O\]
When water acts as an oxidising agent,
it is reduced to\[{{H}_{2}}\].Water reacts with number of active metals whose
electrode potential is less than - 0.83 V.
\[\underset{\text{Reductant}}{\mathop{2Na+}}\,\underset{Oxidant}{\mathop{2{{H}_{2}}O}}\,\to
2NaOH+{{H}_{2}}\]
You need to login to perform this action.
You will be redirected in
3 sec
