Answer:
Autoprotolysis is a reaction in
which two same molecules react to give ions with proton transfer.
Water undergoes autoprotolysis,
i.e., a proton from one molecule is transferred to another molecule.
\[{{H}_{2}}O(l)+{{H}_{2}}O(l)\underset{{}}{\leftrightarrows}{{H}_{3}}{{O}^{+}}(aq.)+O{{H}^{-}}(aq)\]
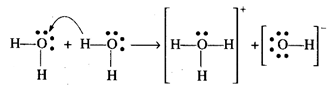 On account of autoprotolysis,
water is amphoteric in nature.
On account of autoprotolysis,
water is amphoteric in nature.
You need to login to perform this action.
You will be redirected in
3 sec
