Answer:
Water is a bent molecule with
bond angle of\[104.5{}^\circ \]. The electro negativity of oxygen is 3.5 and
that of hydrogen 2.1 and hence the shared pair of electrons in the O-H bonds are
attracted slightly more towards the oxygen atom. As a result, oxygen carries a
partial negative charge and hydrogen partial positive charge. Since these two
dipoles are inclined to each other at an angle of\[104.5{}^\circ \]. Therefore,
\[{{H}_{2}}O\]is a polar
molecule.
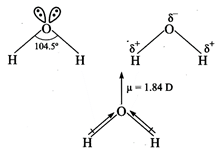 The actual dipole moment of \[{{H}_{2}}O\]
molecule is 1.84 D.
The actual dipole moment of \[{{H}_{2}}O\]
molecule is 1.84 D.
You need to login to perform this action.
You will be redirected in
3 sec
