Answer:
\[\underset{\begin{smallmatrix}
(A) \\
Alkyl\,halide
\end{smallmatrix}}{\mathop{{{C}_{5}}{{H}_{11}}Br}}\,\xrightarrow[\Delta
]{alc.KOH}\underset{\underset{Alkene}{\mathop{(B)}}\,}{\mathop{{{C}_{5}}{{H}_{10}}}}\,\xrightarrow[C{{S}_{2}}]{B{{r}_{2}}}\underset{\underset{Dibromo\,alkane}{\mathop{(C)}}\,}{\mathop{{{C}_{5}}{{H}_{10}}B{{r}_{2}}}}\,\]
\[\xrightarrow[\Delta
]{alc.\,KOH}\underset{\underset{Alkyne}{\mathop{(D)}}\,}{\mathop{{{C}_{5}}{{H}_{8}}}}\,\xrightarrow{{{H}_{2}}}\underset{(Straight\,chain\,alkane)}{\mathop{{{C}_{5}}{{H}_{12}}}}\,\]
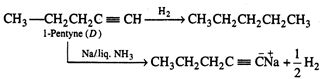
(D)\[\xrightarrow{Na/liqN{{H}_{3}}}{{C}_{5}}H_{7}^{+}N{{a}^{+}}+\frac{1}{2}{{H}_{2}}\]
Hydrogenation of alkyne (D)
gives straight chain alkane hence all the compounds (A), (B), (C) and (D) must
be straight chain compounds. Alkyne (D) forms sodium salt which proves that it
is terminal alkyne. Involved reactions are given below:
\[C{{H}_{3}}-\underset{1-\text{Bromopentane(A)}}{\mathop{C{{H}_{2}}CHL2CHL2}}\,-C{{H}_{2}}Br\xrightarrow[-HBr]{alc.\,KOH}\]
\[C{{H}_{3}}-\underset{1-Pentene(B)}{\mathop{C{{H}_{2}}C{{H}_{2}}CH}}\,=C{{H}_{2}}\xrightarrow[C{{S}_{2}}]{B{{r}_{2}}}\]
\[\underset{1,2-\text{Dibromopentane}(C)}{\mathop{C{{H}_{3}}-C{{H}_{2}}C{{H}_{2}}\overset{\overset{Br}{\mathop{|}}\,}{\mathop{CH}}\,-\overset{\overset{Br}{\mathop{|}}\,}{\mathop{C{{H}_{2}}}}\,}}\,\xrightarrow[-2HBr]{alc.\,KOH}\]
You need to login to perform this action.
You will be redirected in
3 sec
