Answer:
(A) The compound has \[8\pi \]-electrons,
it will be non aromatic; both rings are non-benzenoid.
(B)It is aromatic. It has \[6\pi
\]-delocalised electrons\[(4\pi {{e}^{-}}+2\] lone pair electron)
(C) 6\[\pi \]-electrons but not
in the ring hence it is non-aromatic
(D) 10\[\pi {{e}^{-}}\] obeying
Huckel rule. It is aromatic.
(E) 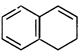 one ring has \[6\pi
{{e}^{-}}\]. It is therefore aromatic.
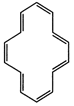
(F) It has \[14\pi \]-electrons
in conjugation and in the ring. Huckel rule is verified. It will be aromatic
provided it has planar ring.
one ring has \[6\pi
{{e}^{-}}\]. It is therefore aromatic.
(F) It has \[14\pi \]-electrons
in conjugation and in the ring. Huckel rule is verified. It will be aromatic
provided it has planar ring.
You need to login to perform this action.
You will be redirected in
3 sec
