Answer:
\[B{{F}_{3}}\] acts as electron pair acceptor, hence it is
Lewis Acid. Coordinate bond is formed between \[B{{F}_{3}}\] and\[N{{H}_{3}}\].
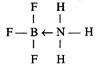
You need to login to perform this action.
You will be redirected in
3 sec
