Answer:
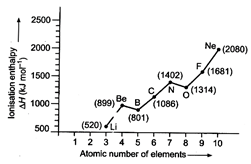
The ionisation enthalpy in general in a
period increases. However, in the given figure deviations are observed between
Be and B and N and O. Be and N both possess stable configurations. Be has fully
filled orbital while N has half filled 2p-orbitals. On account of this Be and N
both have abnormal high values.
You need to login to perform this action.
You will be redirected in
3 sec
