Answer:
(b)
Formal charge of the atom in the molecule or ion =(Number of valence electrons
in free atom) - (Number of lone pair electrons + \[\frac{1}{2}\] Number of
bonding electrons)
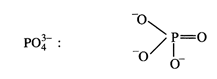 Formal
charge on oxygen =
\[6-\left(
6+\frac{1}{2}\times 2 \right)=6-7=-1\]
Formal
charge on oxygen =
\[6-\left(
6+\frac{1}{2}\times 2 \right)=6-7=-1\]
You need to login to perform this action.
You will be redirected in
3 sec
