Answer:
(c)
Both \[{{H}_{2}}O\] and \[S{{O}_{2}}\] have same shape but the
electro-negativity difference of O and H is more than S and O. Electro
negativity of H, O and S are 2.1, 3.5 and 2.5 respectively.
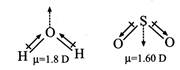
You need to login to perform this action.
You will be redirected in
3 sec
