Answer:
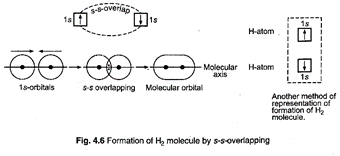
s-s
overlapping (Formation of hydrogen molecule): Each hydrogen atom has one
electron in 1s-orbital which is spherical. 1s-orbital of both the hydrogen atoms
approach each other closely and when they reach a point of maximum attraction
by the two nuclei, they overlap and form a sigma bond.
The
bond has two electrons which have opposite spins.
The
probability of finding these electrons is maximum in the region between the two
nuclei on the molecular axis. The electron density of the bond is distributed symmetrically
about the molecular axis.
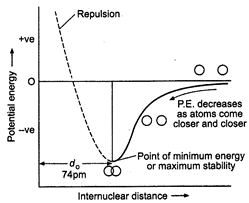
 Variation
of potential energy of interaction between two hydrogen atoms with the inter nuclear
distance may be given as :
Variation
of potential energy of interaction between two hydrogen atoms with the inter nuclear
distance may be given as :
You need to login to perform this action.
You will be redirected in
3 sec
