Answer:
Carbonate
ion can be represented by the following Lewis structure.
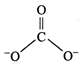 The
calculated values of bond distances between carbon and oxygen in C = 0 and C-0
are 1.22\[\overset{{}^\circ }{\mathop{\text{A}}}\,\] and 1.43\[\overset{{}^\circ
}{\mathop{\text{A}}}\,\]respectively but the observed bond length is neither
1.22 nor 1.43\[\overset{{}^\circ }{\mathop{\text{A}}}\,\] but some what between
these two values. The other observation is that all the three bond lengths are equal.
Hence, the above single structure cannot explain the characteristics of the
carbonate ion. It is, thus, said that resonance exists in this ion and there is
one hybrid form which can provide the exact explanation but its exact structure
cannot be written on paper. Carbonate ion is actually a resonance hybrid of the
following forms :
The
calculated values of bond distances between carbon and oxygen in C = 0 and C-0
are 1.22\[\overset{{}^\circ }{\mathop{\text{A}}}\,\] and 1.43\[\overset{{}^\circ
}{\mathop{\text{A}}}\,\]respectively but the observed bond length is neither
1.22 nor 1.43\[\overset{{}^\circ }{\mathop{\text{A}}}\,\] but some what between
these two values. The other observation is that all the three bond lengths are equal.
Hence, the above single structure cannot explain the characteristics of the
carbonate ion. It is, thus, said that resonance exists in this ion and there is
one hybrid form which can provide the exact explanation but its exact structure
cannot be written on paper. Carbonate ion is actually a resonance hybrid of the
following forms :
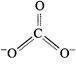
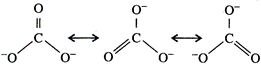 The
resonance hybrid can be best represented as :
The
resonance hybrid can be best represented as :
You need to login to perform this action.
You will be redirected in
3 sec
