Answer:
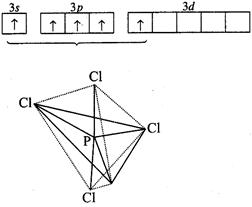
P-atom
has the configuration :
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3p_{x}^{1}3p_{y}^{1}3p_{z}^{1}\]
To
get pentavalency, \[3s\]-orbital is unpaired and electron is shifted to 3d. In
this excited state, the five orbitals undergo hybridisation (\[s{{p}^{3}}d\])
giving rise to trigonal bipyramidal structure. All the five hybrid orbitals
overlap with five chlorine atoms and form sigma bonds.
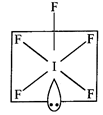
 \[I{{F}_{5}}\]
is square pyramidal. \[I\]-atom have \[{{s}^{2}}{{p}^{5}}\] outer most
configuration. Two \[p\]-orbitals are unpaired and electrons are shifted to \[5d\]-orbitals.
In the excited state \[s{{p}^{3}}{{d}^{2}}-\]hybridisation takes place giving
birth to six hybrid orbitals, i.e., octahedral structure. Five orbitals overlap
with five fluorine atoms and one orbital is occupied by lone pair, i.e., square
pyramidal structure comes into existence.
\[I{{F}_{5}}\]
is square pyramidal. \[I\]-atom have \[{{s}^{2}}{{p}^{5}}\] outer most
configuration. Two \[p\]-orbitals are unpaired and electrons are shifted to \[5d\]-orbitals.
In the excited state \[s{{p}^{3}}{{d}^{2}}-\]hybridisation takes place giving
birth to six hybrid orbitals, i.e., octahedral structure. Five orbitals overlap
with five fluorine atoms and one orbital is occupied by lone pair, i.e., square
pyramidal structure comes into existence.
You need to login to perform this action.
You will be redirected in
3 sec
