Answer:
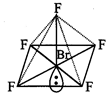
Shape
of \[\mathbf{Br}{{\mathbf{F}}_{\mathbf{5}}}\]
Br-atom
has configuration :
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{10}},4{{s}^{2}}4{{p}^{5}}\]
To
get pentavalency, two of the p-orbitals are unpaired and electrons are shifted
to 4d-orbitals.
 In
this excited state \[s{{p}^{3}}{{d}^{2}}\]-hybridisation occurs giving octahedral
structure. Five positions are occupied by F atoms forming sigma bonds with
hybrid bonds and one position occupied by lone pair i.e., the molecule as a
square pyramidal shape.
In
this excited state \[s{{p}^{3}}{{d}^{2}}\]-hybridisation occurs giving octahedral
structure. Five positions are occupied by F atoms forming sigma bonds with
hybrid bonds and one position occupied by lone pair i.e., the molecule as a
square pyramidal shape.
You need to login to perform this action.
You will be redirected in
3 sec
