question_answer 1) A coil of resistance 10\[\Omega \] and an inductance 5 H is connected to a 100 V battery. The energy stored in the coil is
A)
325 erg
done
clear
B)
125 J
done
clear
C)
250 erg
done
clear
D)
250 J
done
clear
View Answer play_arrow
question_answer 2) A simple pendulum of length; has a brass bob attached at its lower end. Its period is T. If a steel bob of same size, having density \[x\] times that of brass, replaces the brass bob and its length is changed, so that period becomes, 2T then new length is
A)
\[2l\]
done
clear
B)
\[4l\]
done
clear
C)
\[4lx\]
done
clear
D)
\[\frac{41}{x}\]
done
clear
View Answer play_arrow
question_answer 3) Mass of 2 kg is moving with a string in horizontal circle with angular velocity 5 cycles/min keeping the radius constant, tension in string is doubled. Now, the angular velocity of the mass will be
A)
14 cycles/min
done
clear
B)
10 cycles/min
done
clear
C)
2.25 cycles/min
done
clear
D)
7 cycles/min
done
clear
View Answer play_arrow
question_answer 4) The equation of motion for a body executing SHM is given by\[y=1.5\text{ }sin(10~+\text{ }5)\]. Then the frequency is given by
A)
5\[\pi \] Hz
done
clear
B)
1.5 Hz
done
clear
C)
5 Hz
done
clear
D)
\[\pi \]Hz
done
clear
View Answer play_arrow
question_answer 5) A black body emits heat at the rate of 20 W when its temperature is\[727{}^\circ C\]. Another black body emits heat at the rate of 15 W when its temperature is\[227{}^\circ C\]. Compare the area of the surface of the two bodies if the surrounding is at NTP.
A)
16 : 1
done
clear
B)
1 : 4
done
clear
C)
12 : 1
done
clear
D)
1 : 12
done
clear
View Answer play_arrow
question_answer 6) In order to double the separation between the molecules (keeping temperature fixed), the final pressure must be made how many times the initial pressure?
A)
Halved
done
clear
B)
1/4 A
done
clear
C)
1/8 th
done
clear
D)
1/16 th
done
clear
View Answer play_arrow
question_answer 7) What is the potential energy of the soap film formed on a frame of area\[4\times {{10}^{-3}}{{m}^{2}}\]? If the area of the film is reduced by half, the change in its potential energy will be (Surface tension of the soap solution is\[{{10}^{-3}}N/m\])
A)
\[32\times {{10}^{-5}}J,\text{ }16\times {{10}^{-5}}J\]
done
clear
B)
\[16\times {{10}^{-5}}J\text{ }8\times {{10}^{-5}}J\]
done
clear
C)
\[48\times {{10}^{-5}}J,\text{ }12\times {{10}^{-5}}J,\]
done
clear
D)
\[36\times {{10}^{-5}}J\text{ }2\times {{10}^{-5}}J\]
done
clear
View Answer play_arrow
question_answer 8) A body rolls without slipping. The radius of gyration of the body about an axis passing through its centre of mass is K. The radius of the body is R. The ratio of rotational kinetic energy to translational kinetic energy is
A)
\[\frac{{{K}^{2}}}{{{R}^{2}}}\]
done
clear
B)
\[\frac{{{R}^{2}}}{{{K}^{2}}+{{R}^{2}}}\]
done
clear
C)
\[\frac{{{R}^{2}}}{{{K}^{2}}+{{R}^{2}}}\]
done
clear
D)
\[{{K}^{2}}+{{R}^{2}}\]
done
clear
View Answer play_arrow
question_answer 9) In Metre Bridge or Wheatstone bridge for measurement of resistance, the known and the unknown resistances are interchanged. The error so removed is
A)
end correction
done
clear
B)
index error
done
clear
C)
due to temperature effect
done
clear
D)
random error
done
clear
View Answer play_arrow
question_answer 10) The compressibility of water is\[4\times {{10}^{-5}}\]per unit atmospheric pressure, 100 cc of water is subjected to a change of pressure of 100 atmosphere. The change in volume will be
A)
\[4\times {{10}^{-1}}cc\]
done
clear
B)
\[4\times {{10}^{-4}}cc\]
done
clear
C)
\[4\times {{10}^{-2}}cc\]
done
clear
D)
\[4\times {{10}^{-3}}cc\]
done
clear
View Answer play_arrow
question_answer 11) The gas in a vessel is subjected to a pressure of 20 atm at a temperature\[27{}^\circ C\]. The pressure of the gas in a vessel after one-half of the gas is released from the vessel and the temperature of the remainder is raised by\[50{}^\circ C\] is
A)
8.5 atm
done
clear
B)
10.8 atm
done
clear
C)
11.7 atm
done
clear
D)
17 atm
done
clear
View Answer play_arrow
question_answer 12) A tap supplies water at\[15{}^\circ C\]and another tap connected to geyser supplies water at\[95{}^\circ C\]. How much hot water must be taken so as to get 60 kg of water at\[35{}^\circ C\]?
A)
5kg
done
clear
B)
10kg
done
clear
C)
15 kg
done
clear
D)
20 kg
done
clear
View Answer play_arrow
question_answer 13) The magnifying power of a telescope is 9. Hen it is adjusted for parallel rays, the distance between the objective and the eye-piece is found to be 20 cm. The focal length of the lenses are
A)
18 cm, 2 cm
done
clear
B)
11 cm, 9 cm
done
clear
C)
10 cm, 10 cm
done
clear
D)
11 cm, 5 cm
done
clear
View Answer play_arrow
question_answer 14) If the energy of a 100\[\pi F\] capacitor charged to 6 kV could all be used to lift a 50 kg mass, hat would be the greatest vertical height through which mass could be raised?
A)
0.6 mm
done
clear
B)
3.6 m
done
clear
C)
1.2 mm
done
clear
D)
12 m
done
clear
View Answer play_arrow
question_answer 15) The daniel cell is balanced on 125 cm length of a potentiometer. Now the cell is short circuited by a resistance\[2\,\Omega \] and the balance is obtained at 100 cm. The internal resistances of the Daniel cell is
A)
\[\frac{4}{5}\Omega \]
done
clear
B)
1.5 \[\Omega \]
done
clear
C)
1.25 \[\Omega \]
done
clear
D)
0.5\[\Omega \]
done
clear
View Answer play_arrow
question_answer 16) Among which the magnetic susceptibility does not depend on the temperature?
A)
Diamagnetism
done
clear
B)
Paramagnetism
done
clear
C)
Ferromagnetism
done
clear
D)
Ferrite
done
clear
View Answer play_arrow
question_answer 17) A galvanometer has a current range of 15 mA and voltage range 750 mV. To convert this galvanometer into an ammeter of range 25 A, the shunt required is
A)
0.8 \[\Omega \]
done
clear
B)
0.93 \[\Omega \]
done
clear
C)
0.03 \[\Omega \]
done
clear
D)
2.0 \[\Omega \]
done
clear
View Answer play_arrow
question_answer 18) In an atom, two electrons move around the nucleus in circular orbits or radii R and 4R. The ratio of the times taken by them to complete one revolution will be
A)
8 :1
done
clear
B)
1 : 4
done
clear
C)
4 : 1
done
clear
D)
1: 8
done
clear
View Answer play_arrow
question_answer 19) The moment of magnet is\[0.1\text{ }A-{{m}^{2}}\]and the force acting on each pole in a uniform magnetic field of 0.36 oersted is\[1.44\times {{10}^{-4}}\] N. The distance between the poles of magnet is
A)
2.5 cm
done
clear
B)
5.0 cm
done
clear
C)
1.25cm
done
clear
D)
1.17cm
done
clear
View Answer play_arrow
question_answer 20) 1 kg of water is evaporated from 6 kg of sea water containing 4% salt. The percentage of salt in the remaining solution will be
A)
1%
done
clear
B)
3.33%
done
clear
C)
4%
done
clear
D)
4.8%
done
clear
View Answer play_arrow
question_answer 21) A certain radioactive substance has a half-life of 5 years. Thus, for a nucleus in a sample of the element, the probability of decay in 10 years is
A)
50%
done
clear
B)
75%
done
clear
C)
100%
done
clear
D)
60%
done
clear
View Answer play_arrow
question_answer 22) An L-C resonant circuit contains a 400 pF capacitor and a 100 \[\mu H\] inductor. It is set in to oscillation coupled to an antenna. the wavelength of the radiated electromagnetic waves is
A)
377 mm
done
clear
B)
377 m
done
clear
C)
377 cm
done
clear
D)
3.77 cm
done
clear
View Answer play_arrow
question_answer 23) Four resistance of 10\[\Omega \], 60 \[\Omega \] 100 \[\Omega \] and 200 \[\Omega \] respectively taken in order are used to form a. Wheatstones bridge. A 15 V battery is connected to the ends of a 200\[\Omega \], resistance the current through it will be
A)
\[7.5\times {{10}^{-5}}A\]
done
clear
B)
\[7.5\times {{10}^{-4}}A\]
done
clear
C)
\[7.5\times {{10}^{-3}}A\]
done
clear
D)
\[7.5\times {{10}^{-2}}A\]
done
clear
View Answer play_arrow
question_answer 24) A reversible engine working between the temperature limits of 600 K and 1200 K receives 50 kJ of heat. The work done by the engine will be
A)
50 kJ
done
clear
B)
100 kJ
done
clear
C)
25 kJ
done
clear
D)
\[-25\text{ }kJ\]
done
clear
View Answer play_arrow
question_answer 25) Platinum and silicon are heated up to\[250{}^\circ C\] and after that cooled. In this process of cooling
A)
resistance of platinum will increase and that of silicon will decrease
done
clear
B)
resistance of silicon will increase and that of platinum will decrease
done
clear
C)
resistance of both will decrease
done
clear
D)
resistance of both will increase
done
clear
View Answer play_arrow
question_answer 26) A ball after falling freely from a height of 4.9 m strikes a horizontal plane. If the coefficient of restitution is\[\frac{3}{4},\]the ball will strike second time 4 with the plane after
A)
\[\frac{1}{2}s\]
done
clear
B)
\[1\,s\]
done
clear
C)
\[\frac{3}{2}s\]
done
clear
D)
\[\frac{3}{4}s\]
done
clear
View Answer play_arrow
question_answer 27) A particle covers equal distance around a circular path, in equal internals of time. Which of the following quantities connected with the motion of the particle remains constant with time?
A)
Velocity
done
clear
B)
Acceleration
done
clear
C)
Speed
done
clear
D)
Displacement
done
clear
View Answer play_arrow
question_answer 28) A 500 kg horse pulls a cart of mass 1500 kg along a level road with an acceleration of\[1\text{ }m/{{s}^{2}}\]. If the coefficient of sliding friction is 0.2, then the horizontal force exerted by the earth on the horse is
A)
300 kg wt
done
clear
B)
400 kg wt
done
clear
C)
500 kg wt
done
clear
D)
600 kg wt
done
clear
View Answer play_arrow
question_answer 29) The transfer ratio \[\beta \] of a transistor is 50. The input resistance of the transistor when used in the common emitter mode is 1 k\[\Omega \]. The peak value of the collector alternating current for an input peak voltage of 0.01 V is
A)
0.25 \[\mu A\]
done
clear
B)
0.01 \[\mu A\]
done
clear
C)
500 \[\mu A\]
done
clear
D)
100 \[\mu A\]
done
clear
View Answer play_arrow
question_answer 30) If an electron enters a magnetic field with its velocity pointing in the same direction as the magnetic field, then the
A)
electron will turn to its right
done
clear
B)
electron will turn to its left
done
clear
C)
velocity of the electron will increase
done
clear
D)
velocity of the electron will remain Unchanged
done
clear
View Answer play_arrow
question_answer 31) Two charges of magnitude\[4\times {{10}^{-3}}C\]and \[6\times {{10}^{-8}}C\]at A and B are 50 cm apart. The electric potential is zero at a point along AB, where distance from A is given by
A)
40 cm
done
clear
B)
20 cm
done
clear
C)
10 cm
done
clear
D)
30 cm
done
clear
View Answer play_arrow
question_answer 32) Two pendulums begin to swing simultaneously. The first pendulum makes 9 full oscillations while the other makes 7 oscillations. The ratio of lengths of the two pendulums is
A)
\[\frac{9}{7}\]
done
clear
B)
\[\frac{7}{9}\]
done
clear
C)
\[\frac{49}{81}\]
done
clear
D)
\[\frac{81}{49}\]
done
clear
View Answer play_arrow
question_answer 33) The temperature of source and sink of a Carnot engine are\[327{}^\circ C\]and\[27{}^\circ C\] respectively. The efficiencies of the engine is
A)
\[1-\left( \frac{27}{327} \right)\]
done
clear
B)
\[\frac{27}{327}\]
done
clear
C)
\[0.5\]
done
clear
D)
\[0.7\]
done
clear
View Answer play_arrow
question_answer 34) The pressure on a square plate is measured by measuring the force on the plate and the length of the sides of the plate by using the formula \[p=\frac{F}{{{l}^{2}}}.\] If the maximum errors in the measurement of force and length are 4% and 2% respectively. Then the maximum error in the measurement of pressure is
A)
1%
done
clear
B)
2%
done
clear
C)
8%
done
clear
D)
10%
done
clear
View Answer play_arrow
question_answer 35) A point initially at rest moves along x-axis. Its acceleration varies with time as\[a=(61+5)\]in m/s. If it starts from origin, the distance covered in 2s is
A)
20m
done
clear
B)
18m
done
clear
C)
16 m
done
clear
D)
25 m
done
clear
View Answer play_arrow
question_answer 36) A glass marble projected horizontally from the top of a table falls at a distance \[x\] from the edge of the table. If h is the height of the table, then the velocity of projection is
A)
\[h\sqrt{\frac{g}{2x}}\]
done
clear
B)
\[x\sqrt{\frac{g}{2h}}\]
done
clear
C)
\[gxh\]
done
clear
D)
\[gx+h\]
done
clear
View Answer play_arrow
question_answer 37) A circuit has a self-inductance of 1H and carries a current of 2A. To prevent sparking, when the circuit is switched off, a capacitor which can withstand 400v is used. The least capacitance of the capacitor connected across the switch must be equal to
A)
50 \[\mu F\]
done
clear
B)
25 \[\mu F\]
done
clear
C)
100 \[\mu F\]
done
clear
D)
12.5 \[\mu F\]
done
clear
View Answer play_arrow
question_answer 38) A man wants to slide down a rope. The breaking load for the rope \[\frac{2}{3}\] rd of the weight of the man. With what minimum acceleration should fireman slide down?
A)
\[\frac{g}{4}\]
done
clear
B)
\[\frac{g}{3}\]
done
clear
C)
\[\frac{2g}{3}\]
done
clear
D)
\[\frac{g}{6}\]
done
clear
View Answer play_arrow
question_answer 39) A particle moving with a velocity equal to 0.4 m/s is subjected to an acceleration of 0.15 \[m/{{s}^{2}}\]for 2 s in a direction at right angles to its direction of motion. The resultant velocity is
A)
0.7 m/s
done
clear
B)
0.5 m/s
done
clear
C)
m/s
done
clear
D)
between 0.7 and 0.1 m/s
done
clear
View Answer play_arrow
question_answer 40) If the amplitude of a wave at a distance r from a point source is A, the amplitude at a distance 2 r will be
A)
\[2A\]
done
clear
B)
A
done
clear
C)
\[\frac{A}{2}\]
done
clear
D)
\[\frac{A}{4}\]
done
clear
View Answer play_arrow
question_answer 41) Mixture used for the tips of match stick is
A)
\[S+K\]
done
clear
B)
\[S{{b}_{2}}{{S}_{3}}+red\,P\]
done
clear
C)
\[\text{KCl}{{\text{O}}_{\text{3}}}\text{+S+white}\,\text{P}\]
done
clear
D)
\[{{\text{K}}_{\text{2}}}\text{C}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\text{+K+S}\]
done
clear
View Answer play_arrow
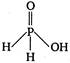
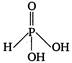
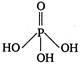
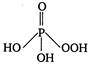
question_answer 42) The structural formula of hypophosphorus acid is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 43) Which of the following has\[p\pi -d\pi \]bonding?
A)
\[NO_{3}^{-}\]
done
clear
B)
\[SO_{3}^{2-}\]
done
clear
C)
\[BO_{3}^{3-}\]
done
clear
D)
\[CO_{3}^{2-}\]
done
clear
View Answer play_arrow
question_answer 44) Ozonised oxygen can be obtained from\[{{H}_{2}}O\]by the action of
A)
conc. \[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[KMn{{O}_{4}}\]
done
clear
C)
\[MnSO_{4}^{2-}\]
done
clear
D)
\[{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 45) Welding of magnesium can be done in an atmosphere of
A)
\[Xe\]
done
clear
B)
He
done
clear
C)
Kr
done
clear
D)
\[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 46) A substance X when heated with cone.\[{{H}_{2}}S{{O}_{4}}\] liberates a gas which turns starch paper blue. The substance X is
A)
\[NaI\]
done
clear
B)
\[NaBr\]
done
clear
C)
\[NaCl\]
done
clear
D)
\[NaN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 47) Sangers reagent is used for the identification of
A)
side chain of amino acids
done
clear
B)
number of amino acids in the peptide chain
done
clear
C)
N-terminal of a peptide chain
done
clear
D)
C-terminal of a peptide chain
done
clear
View Answer play_arrow
question_answer 48) Which of the following is provitamin A?
A)
\[\beta -\]carotene
done
clear
B)
Calciferol
done
clear
C)
Chlorophyll
done
clear
D)
Ergosterol
done
clear
View Answer play_arrow
question_answer 49) When silver nitrate is heated to red hot what is formed?
A)
\[A{{g}_{2}}O\]
done
clear
B)
\[Ag\]
done
clear
C)
\[Ag{{O}_{2}}\]
done
clear
D)
\[AgN{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 50) Which is used as a fungicide?
A)
\[CuS{{O}_{4}}\]and slaked lime
done
clear
B)
\[CuO\] and quick lime
done
clear
C)
\[CuC{{O}_{3}}\]and quick lime
done
clear
D)
\[CuS{{O}_{4}}\]and calcium nitrate
done
clear
View Answer play_arrow
question_answer 51) In the following nuclear reaction, the other product is \[_{52}T{{e}^{130}}{{+}_{1}}{{H}^{2}}{{\xrightarrow[{}]{{}}}_{53}}{{I}^{131}}+?\]
A)
positron
done
clear
B)
one neutron
done
clear
C)
proton
done
clear
D)
alpha particle
done
clear
View Answer play_arrow
question_answer 52) According to MO theory which of the following statements about the magnetic character and bond order is correct regarding\[O_{2}^{+}\]?
A)
Paramagnetic and bond order\[<{{O}_{2}}\]
done
clear
B)
Paramagnetic and bond order\[>{{O}_{2}}\]
done
clear
C)
Diamagnetic and bond order\[<{{O}_{2}}\]
done
clear
D)
Diamagnetic and bond order\[>{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 53) With which of the given pairs\[C{{O}_{2}}\]resembles?
A)
\[HgC{{l}_{2}},{{C}_{2}}{{H}_{2}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{2}},N{{O}_{2}}\]
done
clear
C)
\[HgC{{l}_{2}},\text{ }SnC{{l}_{4}}\]
done
clear
D)
\[{{N}_{2}}O,\text{ }N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 54) The order of magnitude of ionic radii of ions \[N{{a}^{+}},M{{g}^{2+}},A{{l}^{3+}}\]and\[S{{i}^{4+}}\]is
A)
\[N{{a}^{+}}>S{{i}^{4+}}>A{{l}^{3+}}>M{{g}^{2+}}\]
done
clear
B)
\[M{{g}^{2+}}>N{{a}^{+}}>A{{l}^{3+}}>S{{i}^{4+}}\]
done
clear
C)
\[N{{a}^{+}}>M{{g}^{2+}}>A{{l}^{3+}}>S{{i}^{4+}}\]
done
clear
D)
\[S{{i}^{4+}}>A{{l}^{3+}}>M{{g}^{2+}}>N{{a}^{+}}\]
done
clear
View Answer play_arrow
question_answer 55) Which one of the following has Frenkel defect?
A)
\[NaCl\]
done
clear
B)
\[AgBr\]
done
clear
C)
Diamond
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 56) Zn and Hg belong to the same group yet they differ in many of their properties. The property that is shared by both is
A)
they form oxide with air
done
clear
B)
they react with steam readily
done
clear
C)
they react with dil.\[{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
they react with hot\[NaOH\]
done
clear
View Answer play_arrow
question_answer 57) In the extraction of copper from its sulphide ore, the metal is formed by reduction of \[C{{u}_{2}}O\]with
A)
\[FeS\]
done
clear
B)
CO
done
clear
C)
\[C{{i}_{2}}S\]
done
clear
D)
\[S{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 58) N-methylaniline on reaction with nitrous acid gives
A)
N-nitroso-N-methylaniline
done
clear
B)
p-nitroso-N-methylaniline
done
clear
C)
p-nitro-N-methylaniline
done
clear
D)
N-nitro-N-methylaniline
done
clear
View Answer play_arrow
question_answer 59) Which of the following has the highest melting point?
A)
n-pentane
done
clear
B)
Iso-pentane
done
clear
C)
Neo-pentane
done
clear
D)
Cyclopentane
done
clear
View Answer play_arrow
question_answer 60) Identify Z in the following reaction sequence \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\xrightarrow[160-180{}^\circ ]{conc.\,{{H}_{2}}S{{O}_{4}}}X\xrightarrow[{}]{B{{r}_{2}}}\] \[Y\xrightarrow[(ii)\,NaN{{H}_{2}}]{(i)\,Alc.\,LOH}Z\]
A)
\[C{{H}_{3}}CH(N{{H}_{2}})C{{H}_{2}}N{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}CHOHC{{H}_{2}}OH\]
done
clear
C)
\[C{{H}_{3}}\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}C=CH\]
done
clear
View Answer play_arrow
question_answer 61) The hydrogen bond is strongest in
A)
\[O-H---S\]
done
clear
B)
\[SH---O\]
done
clear
C)
\[FH---F\]
done
clear
D)
\[FH---O\]
done
clear
View Answer play_arrow
question_answer 62) Nitromethane is subjected to the treatment with chlorine in the presence of sodium hydroxide, the main product is
A)
monochloromethane
done
clear
B)
trichloromethane
done
clear
C)
chloropicrin
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 63) According to Freundlich adsorption isotherm at high pressure, the value of\[\frac{x}{m}\]is
A)
directly proportional to pressure
done
clear
B)
inversely proportional to pressure
done
clear
C)
directly proportional to square of pressure
done
clear
D)
independent of pressure
done
clear
View Answer play_arrow
question_answer 64) The difference of water molecules in gypsum and plaster of Paris is
A)
\[\frac{5}{2}\]
done
clear
B)
\[2\]
done
clear
C)
\[\frac{1}{2}\]
done
clear
D)
\[1\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 65) An acidified solution of potassium dichromate is turned green when
A)
hydrogen gas is bubbled through it
done
clear
B)
air is passed through it
done
clear
C)
zinc pieces are added to it
done
clear
D)
zinc chloride is added to it
done
clear
View Answer play_arrow
question_answer 66) The ionisation energy of hydrogen atom in the ground state is\[x\text{ }kJ\]. The energy required for an electron to jump from 2nd orbit to 3rd orbit is
A)
\[\frac{5x}{36}\]
done
clear
B)
\[5x\]
done
clear
C)
\[7.2\,x\]
done
clear
D)
\[\frac{x}{6}\]
done
clear
View Answer play_arrow
question_answer 67) At a given temperature, the equilibrium constants for the reactions, \[NO(g)+\frac{1}{2}{{O}_{2}}(g)N{{O}_{2}}(g)\] and \[2N{{O}_{2}}(g)2NO(g)+{{O}_{2}}(g)\] are\[{{K}_{1}}\]and\[{{K}_{2}}\]respectively. If\[{{K}_{1}}\]is\[4\times {{10}^{-3}}\]then will be
A)
\[8\times {{10}^{-3}}\]
done
clear
B)
\[16\times {{10}^{-3}}\]
done
clear
C)
\[6.25\times {{10}^{4}}\]
done
clear
D)
\[6.25\times {{10}^{6}}\]
done
clear
View Answer play_arrow
question_answer 68) At constant T and p which of the following statements is correct for the reaction, \[CO(g)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow[{}]{{}}C{{O}_{2}}(g)?\]
A)
\[\Delta H=\Delta E\]
done
clear
B)
\[\Delta H<\Delta E\]
done
clear
C)
\[\Delta H>\Delta E\]
done
clear
D)
\[\Delta H\]is independent of physical state of reactants
done
clear
View Answer play_arrow
question_answer 69) Sodium metal crystallises in body centred cubic lattice with the cell edge\[a=4.29\overset{o}{\mathop{\text{A}}}\,\]. What is the radius of the sodium atom?
A)
\[1.86\overset{o}{\mathop{\text{A}}}\,\]
done
clear
B)
\[6.81\overset{o}{\mathop{\text{A}}}\,\]
done
clear
C)
\[8.61\overset{o}{\mathop{\text{A}}}\,\]
done
clear
D)
\[2.94\overset{o}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 70) The major product of dehydration of\[{{(C{{H}_{3}})}_{3}}C-C{{H}_{2}}OH\]is
A)
\[{{(C{{H}_{3}})}_{2}}C=CHC{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}CH=CHC{{H}_{3}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}CHCH=C{{H}_{2}}\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}C=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 71) Urea is a diamide of
A)
carbonic acid
done
clear
B)
acetic acid
done
clear
C)
formic acid
done
clear
D)
acetamide
done
clear
View Answer play_arrow
question_answer 72) The IUPAC name of the compound, \[\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C{{H}_{2}}}}\,-\underset{\begin{smallmatrix} | \\ N{{H}_{2}} \end{smallmatrix}}{\mathop{CH}}\,-COOH\]is
A)
2-amino-3-hydroxypropanoic acid
done
clear
B)
1-hydroxy-2-aminopropan-3-oicacid
done
clear
C)
1-amino-2-hydroxypropanoic acid
done
clear
D)
3-hydroxy-2-aminopropanoic acid
done
clear
View Answer play_arrow
question_answer 73) Which one of the following is the stablest structure of cyclohexatriene?
A)
Chair form
done
clear
B)
Boat form
done
clear
C)
Half chair form
done
clear
D)
Planar form
done
clear
View Answer play_arrow
question_answer 74) Which of the following is the most reactive towards ring nitration?
A)
o-xylene
done
clear
B)
Toluene
done
clear
C)
p-xylene
done
clear
D)
m-xylene
done
clear
View Answer play_arrow
question_answer 75) One of the following is a Bronsted acid but not a Bronsted base
A)
\[{{H}_{2}}S\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[HC{{O}_{3}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 76) The pH of a solution obtained by mixing 50 mL of\[0.4\text{ }N\text{ }HCl\]and 50 mL of\[0.2\text{ }N\text{ }NaOH\]is
A)
\[-log\text{ }2\]
done
clear
B)
\[-log\text{ 0}.2\]
done
clear
C)
1.0
done
clear
D)
2.0
done
clear
View Answer play_arrow
question_answer 77) In the cell,\[Zn|Z{{n}^{2+}}({{C}_{1}})||C{{u}^{2+}}({{C}_{2}})|Cu\] \[{{E}_{cell}}-E_{cell}^{o}=0.0591\,V.\]The ratio\[\frac{{{C}_{1}}}{{{C}_{2}}}\]at 298 K will be
A)
2
done
clear
B)
\[{{10}^{-2}}\]
done
clear
C)
1
done
clear
D)
100
done
clear
View Answer play_arrow
question_answer 78) If three faradays of electricity is passed through the solutions of\[AgN{{O}_{3}},CuS{{O}_{4}}\]and \[AuC{{l}_{3}},\]the molar ratio of the cations deposited at the cathode will be
A)
\[1:1:1\]
done
clear
B)
\[1:2:3\]
done
clear
C)
\[3:2:1\]
done
clear
D)
\[6:3:2\]
done
clear
View Answer play_arrow
question_answer 79) The rms velocity of\[C{{O}_{2}}\]at a temperature T (in K) is\[x\text{ }cm\text{ }{{s}^{-1}}\]. At what temperature (in K) the rms velocity of nitrous oxide would be\[4x\text{ }cm{{s}^{-1}}\]?
A)
16 T
done
clear
B)
2T
done
clear
C)
4T
done
clear
D)
32 T
done
clear
View Answer play_arrow
question_answer 80) The oxidation number and covalency of sulphur in sulphur molecule\[({{S}_{8}})\]are
A)
0 and 2
done
clear
B)
+6 and 8
done
clear
C)
0 and 8
done
clear
D)
+6 and 2
done
clear
View Answer play_arrow
question_answer 81) Which of the following statements is correct? Dielectric constant of\[{{H}_{2}}{{O}_{2}}\]
A)
increases with dilution
done
clear
B)
decreases with dilution
done
clear
C)
is unaffected by dilution
done
clear
D)
first increases and then decreases with dilution
done
clear
View Answer play_arrow
question_answer 82) The alumina-silica clay called bentonite is dropped from aeroplanes in the slurry form for
A)
fertilizing the soil
done
clear
B)
spreading water over fires
done
clear
C)
cooling the soil
done
clear
D)
fumigation
done
clear
View Answer play_arrow
question_answer 83) Which of the following on heating does not form an anhydride?
A)
Oxalic acid
done
clear
B)
Succinic acid
done
clear
C)
Maleic acid
done
clear
D)
Glutamic acid
done
clear
View Answer play_arrow
question_answer 84) Which of the following trihalides of P has maximum bond angle?
A)
\[P{{F}_{3}}\]
done
clear
B)
\[PB{{r}_{3}}\]
done
clear
C)
\[PC{{l}_{3}}\]
done
clear
D)
\[P{{I}_{3}}\]
done
clear
View Answer play_arrow
question_answer 85)
\[{{C}_{6}}{{H}_{5}}NM{{e}_{2}}+HCONM{{e}_{2}}\xrightarrow[{}]{POC{{l}_{3}}}\]
A)
Hofmann mustard oil reaction
done
clear
B)
Vilsmeier-Haack reaction
done
clear
C)
Darzens reaction
done
clear
D)
Arndt-Eistert reaction
done
clear
View Answer play_arrow
question_answer 86) A typical compound which undergoes both Cannizaro reaction and aldol condensation is
A)
\[{{(C{{H}_{3}})}_{2}}CH-CHO\]
done
clear
B)
\[HCHO\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}CHO\]
done
clear
D)
\[C{{H}_{3}}CHO\]
done
clear
View Answer play_arrow
question_answer 87) Which of the following sets of reactants is used for the preparation of paracetamol from phenol?
A)
\[HN{{O}_{3}},{{H}_{2}}/Pd,{{(C{{H}_{3}}CO)}_{2}}O\]
done
clear
B)
\[{{H}_{2}}S{{O}_{4}},{{H}_{2}}/Pd,{{(C{{H}_{3}}CO)}_{2}}O\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}{{N}_{2}}Cl,SnC{{l}_{2}}/HCl,(C{{H}_{3}}C{{O}_{2}})O\]
done
clear
D)
\[B{{r}_{2}}/{{H}_{2}}O,Zn/HCl,{{(C{{H}_{3}}CO)}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 88) Ethyl acetate is obtained when methyl magnesium bromide reacts with
A)
ethyl formate
done
clear
B)
acetyl chloride
done
clear
C)
carbon dioxide
done
clear
D)
ethyl chloroformate
done
clear
View Answer play_arrow
question_answer 89) 150 mL of\[{{C}_{2}}HOH\](density 0.78 g/mL) is diluted to 1 L by adding water. Molality of the solution is
A)
2.54
done
clear
B)
1.17
done
clear
C)
2.99
done
clear
D)
29.9
done
clear
View Answer play_arrow
question_answer 90) Which of the following does not have optical isomer?
A)
\[[Co(en){{(N{{H}_{3}})}_{2}}C{{l}_{2}}]Cl\]
done
clear
B)
\[[Co{{(en)}_{2}}C{{l}_{2}}]Cl\]
done
clear
C)
\[[Co{{(N{{H}_{3}})}_{3}}C{{l}_{3}}]\]
done
clear
D)
\[[Co{{(en)}_{3}}]C{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 91) With oxalic acid, glycerol at\[260{}^\circ C\]gives
A)
formic acid
done
clear
B)
allyl alcohol
done
clear
C)
acrolein
done
clear
D)
glyceraldehyde
done
clear
View Answer play_arrow
question_answer 92) When phenolic ether is heated with HI, it yields
A)
alkyl halide + aryl halide + water
done
clear
B)
alkyl halide + phenol
done
clear
C)
alcohol + aryl halide
done
clear
D)
aryl halide + phenol
done
clear
View Answer play_arrow
question_answer 93) The rate constant for the reaction, \[2{{N}_{2}}{{O}_{5}}\xrightarrow[{}]{{}}4N{{O}_{2}}+{{O}_{2}}\] is\[3.0\times {{10}^{-5}}{{s}^{-1}}\]. If the rate is\[2.40\times {{10}^{-5}}\]then the concentration of\[{{N}_{2}}{{O}_{5}}\](in mol/L) is
A)
1.4
done
clear
B)
1.2
done
clear
C)
0.04
done
clear
D)
0.8
done
clear
View Answer play_arrow
question_answer 94) Permanent hardness of water can be removed by adding
A)
sodium chloride
done
clear
B)
soda lime
done
clear
C)
sodium carbonate
done
clear
D)
sodium sulphate
done
clear
View Answer play_arrow
question_answer 95) The pyrimidine bases present in DNA are
A)
cytosine and adenine
done
clear
B)
cytosine and guanine
done
clear
C)
cytosine and thymine
done
clear
D)
cytosine and uracil
done
clear
View Answer play_arrow
question_answer 96) Arsenic drugs are mainly used in the treatment of
A)
jaundice
done
clear
B)
typhoid
done
clear
C)
cholera
done
clear
D)
syphilis
done
clear
View Answer play_arrow
question_answer 97) Which of the following is a secondary pollutant?
A)
NO
done
clear
B)
CO
done
clear
C)
\[S{{O}_{2}}\]
done
clear
D)
Phenols
done
clear
View Answer play_arrow
question_answer 98) For a chemical reaction,.........can never be fraction.
A)
order
done
clear
B)
half-life
done
clear
C)
molecularity
done
clear
D)
rate constant
done
clear
View Answer play_arrow
question_answer 99) Which of the following has\[s{{p}^{2}}-\]hybridisation?
A)
\[S{{O}_{2}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[SO_{4}^{2-}\]
done
clear
View Answer play_arrow
question_answer 100) Acetic acid dissolved in benzene show a molecular mass of
A)
30
done
clear
B)
60
done
clear
C)
120
done
clear
D)
180
done
clear
View Answer play_arrow
question_answer 101) Wild rice originated in
A)
valley to Kashmir
done
clear
B)
coast of Arabian Sea
done
clear
C)
coast of Bay of Bengal
done
clear
D)
delta of River Ganges
done
clear
View Answer play_arrow
question_answer 102) Who is the famous Indian agriculturist. Scientist of International repute?
A)
B P Pal
done
clear
B)
V L Chopra
done
clear
C)
E A Siddiqui
done
clear
D)
M S Swaminathan
done
clear
View Answer play_arrow
question_answer 103) Mark the odd one.
A)
Family
done
clear
B)
Class
done
clear
C)
Taxon
done
clear
D)
Phylum
done
clear
View Answer play_arrow
question_answer 104) With which one of the following a bacterium resembles most?
A)
Yeast
done
clear
B)
Virus
done
clear
C)
Amoeba
done
clear
D)
Anabaena
done
clear
View Answer play_arrow
question_answer 105) Gymnosperms lack
A)
herbs
done
clear
B)
shrubs
done
clear
C)
trees
done
clear
D)
lianas
done
clear
View Answer play_arrow
question_answer 106) Thalamiflorae, Calyciflorae and Disciflorae are series of
A)
Polypetalae
done
clear
B)
Gamopetalae
done
clear
C)
Monochlamydae
done
clear
D)
Monocots
done
clear
View Answer play_arrow
question_answer 107) Cyanophycean algae are much important from point of view of
A)
food and fodder value
done
clear
B)
medicinal value
done
clear
C)
fibres and oils
done
clear
D)
oils fertility
done
clear
View Answer play_arrow
question_answer 108) Gill of mushrooms are meant for
A)
reproduction
done
clear
B)
respiration
done
clear
C)
assimilation
done
clear
D)
nutrition
done
clear
View Answer play_arrow
question_answer 109) Monoecious mosses in which antheridia and archegonia are borne on separate branches of the same plant is called
A)
synoecious mosses
done
clear
B)
heteroecious mosses
done
clear
C)
autoecious mosses
done
clear
D)
autoecious mosses
done
clear
View Answer play_arrow
question_answer 110) Formation of sex cells was first seen in
A)
pteridophytes
done
clear
B)
bryophytes
done
clear
C)
gymnosperms
done
clear
D)
angiosperms
done
clear
View Answer play_arrow
question_answer 111) Multicellular spores are released from the capsule of
A)
Riccia, Marchantia
done
clear
B)
Pellia, Porella
done
clear
C)
Anthoceros, Notothylas
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 112) Which of the following can be seen only under the electron microscope?
A)
Ribosomes
done
clear
B)
Leucoplasts
done
clear
C)
Chloroplasts
done
clear
D)
Chromosomes
done
clear
View Answer play_arrow
question_answer 113) Geiger-Muller counter is used for the detection and measurement of
A)
RBCs
done
clear
B)
WBCs
done
clear
C)
radiation
done
clear
D)
amino acids
done
clear
View Answer play_arrow
question_answer 114) Golgi apparatus is absent in
A)
yeast
done
clear
B)
liver cells
done
clear
C)
higher plants
done
clear
D)
blue-green algae
done
clear
View Answer play_arrow
question_answer 115) Which of the following algae exhibits gametic meiosis?
A)
Chara
done
clear
B)
Sargassam
done
clear
C)
Polysiphonia
done
clear
D)
Oedogonium
done
clear
View Answer play_arrow
question_answer 116) Which one is not a carbohydrate?
A)
Starch
done
clear
B)
Chitin
done
clear
C)
Glycogen
done
clear
D)
Methionine
done
clear
View Answer play_arrow
question_answer 117) How many trisomics are possible in Datura?
A)
10
done
clear
B)
24
done
clear
C)
12
done
clear
D)
30
done
clear
View Answer play_arrow
question_answer 118) Utricularia grows in
A)
evergreen forests
done
clear
B)
lakes and ponds as submerged hydrophyte
done
clear
C)
bog as a marsh plant
done
clear
D)
tropical deciduous forest
done
clear
View Answer play_arrow
question_answer 119) The maize plant is monoecious and the flowers are
A)
cleistogamous
done
clear
B)
unisexual
done
clear
C)
bisexual
done
clear
D)
neutar
done
clear
View Answer play_arrow
question_answer 120) Seed surface produces cellulosic fibres in
A)
Helianthus
done
clear
B)
Taraxacum
done
clear
C)
Gossypium
done
clear
D)
Xanthium
done
clear
View Answer play_arrow
question_answer 121) Perianth is reduced to lodicules in
A)
Liliaceae
done
clear
B)
Poaceae
done
clear
C)
Orchidaceae
done
clear
D)
Musaceae
done
clear
View Answer play_arrow
question_answer 122) Ring porous and diffused porous wood are produced by
A)
conifers
done
clear
B)
angiosperms
done
clear
C)
monocots
done
clear
D)
cycads
done
clear
View Answer play_arrow
question_answer 123) Bast fibres are obtained from
A)
epidermis
done
clear
B)
seed surface
done
clear
C)
phloem
done
clear
D)
pith
done
clear
View Answer play_arrow
question_answer 124) Meristem, which produces vascular bundles, is
A)
procambium
done
clear
B)
lateral meristem
done
clear
C)
secondary meristem
done
clear
D)
mass meristem
done
clear
View Answer play_arrow
question_answer 125) In hypertonic solution, water potential of cell
A)
increases
done
clear
B)
decreases
done
clear
C)
first increases and then decreases
done
clear
D)
remains unchanged
done
clear
View Answer play_arrow
question_answer 126) Enzyme catalyzed reactions can be inhibited by
A)
\[Z{{n}^{2+}}\]
done
clear
B)
\[M{{g}^{2+}}\]
done
clear
C)
\[H{{g}^{2+}}\]
done
clear
D)
\[C{{u}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 127) One of the following amino acids which one is sulphur containing one?
A)
Aspargine
done
clear
B)
Proline
done
clear
C)
Serine
done
clear
D)
Methionine
done
clear
View Answer play_arrow
question_answer 128) Photolysis of water is caused by
A)
PS-I
done
clear
B)
PS-II
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 129) Name the enzyme which changes its characteristics with change in concentration of\[{{O}_{2}}\]?
A)
RuBP carboxylase
done
clear
B)
PEP
done
clear
C)
PGA
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 130) Oxygen produced in photosynthesis comes from \[{{\text{H}}_{2}}\text{O}\] was shown by
A)
Robert Mayer
done
clear
B)
Ruben and Kamen
done
clear
C)
Calvin
done
clear
D)
Robert Hill
done
clear
View Answer play_arrow
question_answer 131) How many ATP and NADH; are produced in photorespiration?
A)
2 and 4
done
clear
B)
1 and 2
done
clear
C)
4 and 6
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 132) Enzymes and hormones both are synthesized from
A)
endothecium
done
clear
B)
inner layer
done
clear
C)
archesporium
done
clear
D)
tapetum
done
clear
View Answer play_arrow
question_answer 133) The ploidy level is not the same in
A)
integument and nucellus
done
clear
B)
root tip and shoot tip
done
clear
C)
secondary nucleus and endosperm
done
clear
D)
antipodals and synergids
done
clear
View Answer play_arrow
question_answer 134) In the reciprocal crosses, the ploidy level does not change in
A)
embryo
done
clear
B)
fruit
done
clear
C)
endosperms
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 135) The material best for micropropagation is
A)
pollen
done
clear
B)
embryo
done
clear
C)
endosperm
done
clear
D)
shoot or root tips
done
clear
View Answer play_arrow
question_answer 136) Cybrids carry
A)
two similar genomes
done
clear
B)
only one genome
done
clear
C)
several genomes
done
clear
D)
one genome and two plasmones
done
clear
View Answer play_arrow
question_answer 137) Apical dominance is not affected by
A)
indole acetic acid
done
clear
B)
gibberellins
done
clear
C)
indole acetaldehyde
done
clear
D)
indole butyric acid
done
clear
View Answer play_arrow
question_answer 138) Sleeping movements in Samanea saman leaves are regulated by
A)
nitrogen
done
clear
B)
potassium
done
clear
C)
phosphorus
done
clear
D)
magnesium
done
clear
View Answer play_arrow
question_answer 139) Maximum amount of growth in root occurs
A)
in the presence of light
done
clear
B)
at its apex
done
clear
C)
behind the apex
done
clear
D)
in the presence of soil
done
clear
View Answer play_arrow
question_answer 140) Ecotypes are
A)
only morphologically different
done
clear
B)
only genetically different
done
clear
C)
Both [a] and [b]
done
clear
D)
both morphologically and genetically similar
done
clear
View Answer play_arrow
question_answer 141) Individuals of one kind occupying a particular geographical area are forming a
A)
community
done
clear
B)
population
done
clear
C)
biome
done
clear
D)
species
done
clear
View Answer play_arrow
question_answer 142) Shifting cultivation is harming much to
A)
biodiversity
done
clear
B)
forestry
done
clear
C)
silviculture
done
clear
D)
horticulture
done
clear
View Answer play_arrow
question_answer 143) Petrol and natural gases are found between the layers of
A)
magnetic rocks
done
clear
B)
volcanic rocks
done
clear
C)
igneous rocks
done
clear
D)
sedimentary rocks
done
clear
View Answer play_arrow
question_answer 144) Interlocking of food chain results into the formation of
A)
ecological pyramids
done
clear
B)
energy flow
done
clear
C)
food web
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 145) Banana is cultivated by
A)
vegetative means
done
clear
B)
bulbs
done
clear
C)
seeds
done
clear
D)
bulbilk
done
clear
View Answer play_arrow
question_answer 146) Resetting of leaves in infected plants refers to
A)
etiolation
done
clear
B)
chlorosis
done
clear
C)
necrosis
done
clear
D)
atrophy
done
clear
View Answer play_arrow
question_answer 147) Plasmids capable of degrading octane, xylene, camphor and naphthalene occur in cells of
A)
Pseudomonas putida
done
clear
B)
Bacillus subtilis
done
clear
C)
Xanthomonas citri
done
clear
D)
E. coli
done
clear
View Answer play_arrow
question_answer 148) Transitional zone between two vegetation types is referred to
A)
ecotone
done
clear
B)
ecotype
done
clear
C)
ecades
done
clear
D)
ecophenes
done
clear
View Answer play_arrow
question_answer 149) Dischidia is a pitcher plant which
A)
shows insectivory
done
clear
B)
shows parasitism
done
clear
C)
shows epiphytic nature
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 150) Auxin (IAA) was isolated by Thimann in 1935 from
A)
bryophytes
done
clear
B)
algae
done
clear
C)
fungi
done
clear
D)
pteridophytes
done
clear
View Answer play_arrow
question_answer 151) Histiocyte is a connective tissue cell which takes part in
A)
secretion
done
clear
B)
phagocytosis
done
clear
C)
fibre production
done
clear
D)
matrix production
done
clear
View Answer play_arrow
question_answer 152) Major cause of anaemia is deficiency of
A)
\[C{{a}^{2+}}\]
done
clear
B)
\[F{{e}^{2+}}\]
done
clear
C)
\[N{{a}^{+}}\]
done
clear
D)
\[M{{g}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 153) Mast cells secrete
A)
heparin
done
clear
B)
histamine
done
clear
C)
serotonin
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 154) Axons serve to
A)
take away impulse from cytons
done
clear
B)
bring impulse to cytons
done
clear
C)
bring blood to heart
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 155) Cerebral hemispheres of rat are connected by
A)
corpus luteum
done
clear
B)
corpus callosum
done
clear
C)
corpus albicans
done
clear
D)
corpus spongiosum
done
clear
View Answer play_arrow
question_answer 156) A renal portal system is found in
A)
man
done
clear
B)
frog
done
clear
C)
horse
done
clear
D)
rabbit
done
clear
View Answer play_arrow
question_answer 157) Rennin is found in
A)
bile
done
clear
B)
saliva
done
clear
C)
gastric juice
done
clear
D)
pancreatic juice
done
clear
View Answer play_arrow
question_answer 158) Absorption of fat-soluble vitamin requires
A)
chemotrypsin
done
clear
B)
intrinsic factor
done
clear
C)
pancreatic lipase
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 159) Rate of breathing is directly attacted by
A)
oxygen in trachea
done
clear
B)
concentration of \[{{O}^{2}}\]
done
clear
C)
concentration of \[C{{O}^{3}}\]
done
clear
D)
diaphragm expansion
done
clear
View Answer play_arrow
question_answer 160) Carotid labyrinth contains
A)
baroreceptors
done
clear
B)
phonoreceptors
done
clear
C)
chaemoreceptors
done
clear
D)
olfactoreceptors
done
clear
View Answer play_arrow
question_answer 161) Lymph capillaries differ from systemic blood capillaries in that they
A)
collapse when interstitial pressure increases
done
clear
B)
are absent in the central nervous system
done
clear
C)
are not lined by endothelium
done
clear
D)
lack valves
done
clear
View Answer play_arrow
question_answer 162) Which of these will be completely reabsorbed from glomerular filtrate under normal conditions in the nephrons?
A)
Urea
done
clear
B)
Salts
done
clear
C)
Glucose
done
clear
D)
Uric acid
done
clear
View Answer play_arrow
question_answer 163) The largest muscle in the human body is
A)
gluteus
done
clear
B)
stapedius
done
clear
C)
sartorius
done
clear
D)
masseter
done
clear
View Answer play_arrow
question_answer 164) In man, coccygeal bone is formed by the fusion of......... vertebrae.
A)
3
done
clear
B)
4
done
clear
C)
5
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 165) Myelin sheath is a layer covering
A)
muscle fibre in a vertebrate
done
clear
B)
nerve fibre in a vertebrate
done
clear
C)
ne rve fibre in an insect
done
clear
D)
chick embryo
done
clear
View Answer play_arrow
question_answer 166) Which one is main fuel for brain?
A)
Glucose
done
clear
B)
Riboflavin
done
clear
C)
Ornithine
done
clear
D)
Glutamic acid
done
clear
View Answer play_arrow
question_answer 167) Which of the following is known as cochlear duct?
A)
Scala media
done
clear
B)
Scala vestibuli
done
clear
C)
Scala tympani
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 168) The adrenal cortex secretes all the following hormones except
A)
tyrosine
done
clear
B)
cholesterol
done
clear
C)
glycoprotein
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 169) Which one is a modified lymph gland?
A)
Pineal
done
clear
B)
Thymus
done
clear
C)
Pituitary
done
clear
D)
Thyroid
done
clear
View Answer play_arrow
question_answer 170) The blood vessel and nerves enter the ovary through
A)
hilus
done
clear
B)
zona pellucida
done
clear
C)
antrum
done
clear
D)
Graafian follicle
done
clear
View Answer play_arrow
question_answer 171) In simplest type of placenta, six barriers separate maternal blood from foetal blood. How many barriers are lost in human placenta?
A)
One
done
clear
B)
Four
done
clear
C)
Two
done
clear
D)
Three
done
clear
View Answer play_arrow
question_answer 172) Decline in hearing power begins in man after
A)
2 years
done
clear
B)
10 years
done
clear
C)
60 years
done
clear
D)
80 years
done
clear
View Answer play_arrow
question_answer 173) The bones of old people become hard and brittle due to deposition of
A)
calcium
done
clear
B)
potassium
done
clear
C)
sodium
done
clear
D)
carbon
done
clear
View Answer play_arrow
question_answer 174) Highest rate of primary productivity is found in
A)
tropical rainforests
done
clear
B)
coral reef and estuaries
done
clear
C)
forest floor
done
clear
D)
warm temperature forests
done
clear
View Answer play_arrow
question_answer 175) Hormone used in sterile cows to induce lactation is
A)
relaxin
done
clear
B)
stilbesterol
done
clear
C)
oestrogen
done
clear
D)
progesterone
done
clear
View Answer play_arrow
question_answer 176) The disease, tetanus is also known as
A)
shingles
done
clear
B)
gangrene
done
clear
C)
lockjaw
done
clear
D)
whooping cough
done
clear
View Answer play_arrow
question_answer 177) Which of the following T-cells are destroyed by HIV?
A)
Cytotoxic T-cells
done
clear
B)
Killer T-cells
done
clear
C)
Suppressor T-cells
done
clear
D)
Helper T-cells
done
clear
View Answer play_arrow
question_answer 178) Humulin is a
A)
natural insulin
done
clear
B)
insulin synthesized by genetically engineered E. coli
done
clear
C)
human insulin synthesized by pancreas
done
clear
D)
chemically synthesized insulin
done
clear
View Answer play_arrow
question_answer 179) In Hydra, sperms and ova are formed from
A)
cnidoblasts
done
clear
B)
interstitial cells
done
clear
C)
epithelio-muscular cells
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 180) How many filaments of Spirogyra are produced by germination of 100 zygospores?
A)
100
done
clear
B)
200
done
clear
C)
400
done
clear
D)
50
done
clear
View Answer play_arrow
question_answer 181) Muscles in sponges are
A)
striated
done
clear
B)
smooth
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 182) Which of these is not a cnidarian?
A)
Jellyfish
done
clear
B)
Silverfish
done
clear
C)
Sea anemone
done
clear
D)
Physalia
done
clear
View Answer play_arrow
question_answer 183) Cirrus in Taenia is associated with
A)
excretion
done
clear
B)
digestion
done
clear
C)
circulation
done
clear
D)
reproduction
done
clear
View Answer play_arrow
question_answer 184) During its migration in host body. Ascents passes through
A)
lungs
done
clear
B)
spleen
done
clear
C)
kidney
done
clear
D)
skeletal muscle
done
clear
View Answer play_arrow
question_answer 185) A definite number of body segments is found in
A)
slug
done
clear
B)
leech
done
clear
C)
earthworm
done
clear
D)
tapeworm
done
clear
View Answer play_arrow
question_answer 186) The outermost covering of the egg of housefly is
A)
chitin
done
clear
B)
chorion
done
clear
C)
vitelline membrane
done
clear
D)
plasma membrane
done
clear
View Answer play_arrow
question_answer 187) Pharyngeal gill-slits are found in
A)
Octopus
done
clear
B)
crayfish
done
clear
C)
dogfish
done
clear
D)
cuttlefish
done
clear
View Answer play_arrow
question_answer 188) Preen gland occurs in
A)
aves
done
clear
B)
pisces
done
clear
C)
reptiles
done
clear
D)
mammals
done
clear
View Answer play_arrow
question_answer 189) Sarcoplasmic reticulum is found in
A)
liver
done
clear
B)
brain
done
clear
C)
muscles
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 190) Select the odd one.
A)
Oleic acid
done
clear
B)
Butyric acid
done
clear
C)
Cupric acid
done
clear
D)
Glutamic acid
done
clear
View Answer play_arrow
question_answer 191) Which one is essential fatty acid having three double bonds?
A)
Linoleic acid
done
clear
B)
Linolenic acid
done
clear
C)
Arachidonic acid
done
clear
D)
Clupanodonic acid
done
clear
View Answer play_arrow
question_answer 192) Amylase is an example of
A)
ligase
done
clear
B)
hydrolase
done
clear
C)
transferase
done
clear
D)
oxidoreductase
done
clear
View Answer play_arrow
question_answer 193) Lipase breaks the
A)
ester bonds
done
clear
B)
peptide bonds
done
clear
C)
hydrogen bonds
done
clear
D)
glycosidic bonds
done
clear
View Answer play_arrow
question_answer 194) A gene, whose phenotypic effect kills the bearer, is called
A)
lethal
done
clear
B)
pleiotropic
done
clear
C)
supplementary
done
clear
D)
complementary
done
clear
View Answer play_arrow
question_answer 195) The segment of a DNA molecule determining the amino acid sequence of a protein, is known as
A)
operator gene
done
clear
B)
regulator gene
done
clear
C)
structural gene
done
clear
D)
modifier gene
done
clear
View Answer play_arrow
question_answer 196) A boy receives his X-chromosome from
A)
his mother only
done
clear
B)
his father only
done
clear
C)
Both father and mother
done
clear
D)
Either mother or father
done
clear
View Answer play_arrow
question_answer 197) The possibilities of hereditary and evolutionary changes are greater in species that are reproduced by
A)
fission
done
clear
B)
budding
done
clear
C)
sexual means
done
clear
D)
spore formation
done
clear
View Answer play_arrow
question_answer 198) The most important theory of general biology was proposed by
A)
Beadle and Tatum
done
clear
B)
Watson and Crick
done
clear
C)
Darwin and Wallace
done
clear
D)
Mendel and Morgan
done
clear
View Answer play_arrow
question_answer 199) Carriers of colour blindness are
A)
men
done
clear
B)
women
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 200) Which of these enzyme is not required for DNA synthesis?
A)
Ligase
done
clear
B)
DNAse
done
clear
C)
DNA polymerase
done
clear
D)
RNA polymerase
done
clear
View Answer play_arrow