question_answer 1) How many seconds are there in a light fermi?
A)
\[{{10}^{-15}}s\]
done
clear
B)
\[3.0\times {{10}^{8}}s\]
done
clear
C)
\[3.33\times {{10}^{-24}}s\]
done
clear
D)
\[3.3\times {{10}^{-7}}s\]
done
clear
View Answer play_arrow
question_answer 2) A machine is delivering constant power to drive a body along a straight line. What is the relation between the distance travelled by the body against time?
A)
\[{{s}^{2}}\propto {{t}^{3}}\]
done
clear
B)
\[{{s}^{2}}\propto {{t}^{-3}}\]
done
clear
C)
\[{{s}^{2}}\propto {{t}^{2}}\]
done
clear
D)
\[s\propto {{t}^{3}}\]
done
clear
View Answer play_arrow
question_answer 3) The square of resultant of two equal forces is three times their product. Angle between the forces is
A)
\[\pi \]
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
\[\frac{\pi }{4}\]
done
clear
D)
\[\frac{\pi }{3}\]
done
clear
View Answer play_arrow
question_answer 4) An object placed on a ground is in stable equilibrium. If the object is given a slight push then initially the position of centre of gravity
A)
moves nearer to ground
done
clear
B)
rises higher above the ground
done
clear
C)
remains as such
done
clear
D)
may remain at same level
done
clear
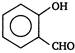
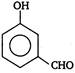
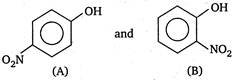
View Answer play_arrow
question_answer 5) How much work must be done by a force on 50 kg body in order to accelerate it from rest to 20 m/s in 10 s?
A)
\[{{10}^{3}}J\]
done
clear
B)
\[{{10}^{4}}J\]
done
clear
C)
\[2\times {{10}^{3}}J\]
done
clear
D)
\[4\times {{10}^{4}}J\]
done
clear
View Answer play_arrow
question_answer 6) Moment of inertia of circular loop of radius R about the axis of rotation parallel to horizontal diameter at a distance R/2 from it is
A)
\[M{{R}^{2}}\]
done
clear
B)
\[\frac{1}{2}M{{R}^{2}}\]MR
done
clear
C)
\[2M{{R}^{2}}\]
done
clear
D)
\[\frac{3}{4}M{{R}^{2}}\]
done
clear
View Answer play_arrow
question_answer 7) What will happen to the weight of the body at the south pole, if the earth stops rotating about its polar axis?
A)
No change
done
clear
B)
Increases
done
clear
C)
Decreases but does not become zero
done
clear
D)
Reduces to zero
done
clear
View Answer play_arrow
question_answer 8) A beam of metal supported at the two ends is loaded at tlie centre. The depression at the centre is proportional to
A)
\[{{Y}^{2}}\]
done
clear
B)
\[Y\]
done
clear
C)
\[\frac{1}{Y}\]
done
clear
D)
\[\frac{1}{{{Y}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 9) A common hydrometer reads specific gravity of liquids. Compared to the 1.6 mark of the stem the mark 1.5 will be
A)
upwards
done
clear
B)
downwards
done
clear
C)
in the same place
done
clear
D)
may be upward or downward depending upon the hydrometer
done
clear
View Answer play_arrow
question_answer 10) A balloon contains\[500\text{ }{{m}^{3}}\]of Heat\[27{}^\circ C\]and 1 atmospheric pressure. The volume of Heat\[-3{}^\circ C\]and 0.5 atmospheric pressure will be
A)
\[700{{m}^{3}}\]
done
clear
B)
\[900{{m}^{3}}\]
done
clear
C)
\[1000{{m}^{3}}\]
done
clear
D)
\[500{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 11) Which of the following is different from others?
A)
Wavelength
done
clear
B)
Velocity
done
clear
C)
Frequency
done
clear
D)
Amplitude
done
clear
View Answer play_arrow
question_answer 12) Two pendulums have time periods T and 5T/4. They starts SHM at the same time from the mean position. What will be the phase difference between them after the bigger pendulum completed one oscillation ?
A)
\[45{}^\circ \]
done
clear
B)
\[90{}^\circ \]
done
clear
C)
\[60{}^\circ \]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
question_answer 13) A balloon is filled with hydrogen. For sound waves, this balloon behaves like
A)
a converging lens
done
clear
B)
a diverging lens
done
clear
C)
a concave mirror
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 14) Each of the two point charges are doubled and their distance is halved. Force of interaction becomes n times, where n is
A)
4
done
clear
B)
1
done
clear
C)
1/16
done
clear
D)
16
done
clear
View Answer play_arrow
question_answer 15) Two soap bubbles have radii in the ratio of 2:1. What is the ratio of excess pressures inside them?
A)
1: 2
done
clear
B)
1: 4
done
clear
C)
2:1
done
clear
D)
4 : 1
done
clear
View Answer play_arrow
question_answer 16) The phenomenon of Brownian movement may be taken as evidence of
A)
kinetic theory of matter
done
clear
B)
EMT of radiation
done
clear
C)
corpuscular theory of light
done
clear
D)
photoelectric phenomenon
done
clear
View Answer play_arrow
question_answer 17) Two sound waves of slightly different frequencies propagating in the same direction produce beats due to
A)
interference
done
clear
B)
diffraction
done
clear
C)
reflection
done
clear
D)
refraction
done
clear
View Answer play_arrow
question_answer 18) An ice block floats in a liquid whose density is less than water. A part of block is outside the liquid. When whole of ice has melted, the liquid level will
A)
rise
done
clear
B)
go down
done
clear
C)
remain same
done
clear
D)
first rise then go down
done
clear
View Answer play_arrow
question_answer 19) Two bodies of different masses of 2 kg and 4 kg moving with velocities 2 m/s and 10 m/s towards each other due to mutual gravitational attraction. What is the velocity of their centre of mass?
A)
5 m/s
done
clear
B)
6 m/s
done
clear
C)
8 m/s
done
clear
D)
Zero
done
clear
View Answer play_arrow
question_answer 20) Given that the displacement of an oscillating particle is given by\[y=Asin(B~+Ct+D)\]. The dimensional formula for (ABCD) is
A)
\[[{{M}_{0}}{{L}^{-1}}{{T}^{0}}]\]
done
clear
B)
\[[M{{\,}^{0}}{{L}^{0}}{{T}^{-1}}]\]
done
clear
C)
\[[{{M}^{0}}{{L}^{-1}}{{T}^{-1}}]\]
done
clear
D)
\[[{{M}^{0}}{{L}^{0}}{{T}^{0}}]\]
done
clear
View Answer play_arrow
question_answer 21) Two waves having intensities in the ratio of 9:1 produce interference. The ratio of maximum to minimum intensity is equal:
A)
10 : 8
done
clear
B)
9 : 1
done
clear
C)
4 :1
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 22) Four wires each of same length, diameter as. materials are connected to each other to form square. If the resistance of each wire is R. then equivalent resistance across the opposite corners is
A)
R
done
clear
B)
R/2
done
clear
C)
R/4
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 23) An electric motor runs on DC source of emf 200 V and draws a current of 10 A. If the efficiency be 40% then the resistance of armature is
A)
20
done
clear
B)
80
done
clear
C)
120
done
clear
D)
160
done
clear
View Answer play_arrow
question_answer 24) A capacitor having capacity of 2.0 \[\mu F\] is charged to 200 V and then the plates of the capacitor are connected to a resistance wire The heat produced in joule will be
A)
\[2\times {{10}^{-2}}\]
done
clear
B)
\[4\times {{10}^{-2}}\]
done
clear
C)
\[4\times {{10}^{4}}\]
done
clear
D)
\[4\times {{10}^{10}}\]
done
clear
View Answer play_arrow
question_answer 25) A voltmeter of range 2V and resistance 300\[\Omega \] cannot be converted into ammeter of range
A)
1A
done
clear
B)
1mA
done
clear
C)
100mA
done
clear
D)
10mA
done
clear
View Answer play_arrow
question_answer 26) If a magnet is suspended at angle\[30{}^\circ \]to the magnetic meridian, the dip needle makes angle of\[45{}^\circ \]with the horizontal. The real dip is
A)
\[{{\tan }^{-1}}(\sqrt{3/2})\]
done
clear
B)
\[{{\tan }^{-1}}(\sqrt{3})\]
done
clear
C)
\[{{\tan }^{-1}}(\sqrt{3/2})\]
done
clear
D)
\[{{\tan }^{-1}}(2/\sqrt{3})\]
done
clear
View Answer play_arrow
question_answer 27) Which quantity is increased in step-down transformer?
A)
Current
done
clear
B)
Voltage
done
clear
C)
Power
done
clear
D)
Frequency
done
clear
View Answer play_arrow
question_answer 28) The ratio of intensity at the centre of a bright fringe to the intensity at a point distant or one fourth of the distance between two successive bright fringes will be
A)
4
done
clear
B)
3
done
clear
C)
2
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 29) Which has more luminous efficiency?
A)
A 40 W bulb
done
clear
B)
A 40 W fluorescent tube
done
clear
C)
Both have same
done
clear
D)
Cannot say
done
clear
View Answer play_arrow
question_answer 30) When a ray of light enters from one medium to another, then its velocity in second medium becomes double. The maximum value of angle of incidence, so that total internal reflection may not take place will be
A)
\[60{}^\circ \]
done
clear
B)
\[180{}^\circ \]
done
clear
C)
\[90{}^\circ \]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
question_answer 31) What should be the velocity of an electron so that its momentum becomes equal to that of a photon of wavelength\[5200\overset{o}{\mathop{\text{A}}}\,\]?
A)
700 m/s
done
clear
B)
1000 m/s
done
clear
C)
1400 m/s
done
clear
D)
2800 m/s
done
clear
View Answer play_arrow
question_answer 32) A radioactive element has half-life period of 600 years. After 3000 years, what amount will remain?
A)
\[\frac{1}{2}\]
done
clear
B)
\[\frac{1}{16}\]
done
clear
C)
\[\frac{1}{8}\]
done
clear
D)
\[\frac{1}{32}\]
done
clear
View Answer play_arrow
question_answer 33) Beyond which frequency, the ionosphere bends any incident electromagnetic radiation but do not reflect it back towards the earth?
A)
50MHz
done
clear
B)
40MHz
done
clear
C)
30MHz
done
clear
D)
20MHz
done
clear
View Answer play_arrow
question_answer 34) In intrinsic semiconductor at room temperature number of electrons and holes are
A)
equal
done
clear
B)
zero
done
clear
C)
unequal
done
clear
D)
infinite
done
clear
View Answer play_arrow
question_answer 35) The unit of thermal conductance is
A)
\[W{{K}^{-1}}\]
done
clear
B)
\[J{{K}^{-1}}\]
done
clear
C)
WK
done
clear
D)
JK
done
clear
View Answer play_arrow
question_answer 36) The value of P so that the vectors \[2\hat{i}-\hat{j}+\hat{k}\hat{,}+2\hat{j}-3\hat{k}\] and \[3\hat{i}+p\hat{j}+5\hat{k}\] are coplanar should be
A)
16
done
clear
B)
\[-4\]
done
clear
C)
4
done
clear
D)
\[-8\]
done
clear
View Answer play_arrow
question_answer 37) A capacitor of capacitance C has charge Q and stored energy is W. If the charge is increased to 2Q, the stored energy will be
A)
\[\frac{W}{4}\]
done
clear
B)
\[\frac{W}{2}\]
done
clear
C)
\[2W\]
done
clear
D)
\[4W\]
done
clear
View Answer play_arrow
question_answer 38) Pure silicon at 300 K has equal electron \[({{n}_{e}})\] and hole \[({{n}_{h}})\] concentration of\[1.5\times {{10}^{16}}{{m}^{-3}}\]. Doping by indium increases \[{{n}_{h}}\] to \[4.5\times {{10}^{22}}{{m}^{-3}}\] . The \[{{n}_{e}}\] in the doped silicon is
A)
\[9\times {{10}^{5}}\]
done
clear
B)
\[5\times {{10}^{9}}\]
done
clear
C)
\[2.25\times {{10}^{11}}\]
done
clear
D)
\[3\times {{10}^{19}}\]
done
clear
View Answer play_arrow
question_answer 39) A cylindrical conductor is placed near another positively charged conductor. The net charge acquired by the cylindrical conductor will be
A)
positive only
done
clear
B)
negative only
done
clear
C)
zero
done
clear
D)
either positive or negative
done
clear
View Answer play_arrow
question_answer 40) If the unit of force is 1 kilo newton, the length is 1 km and time 100 s, what will be the unit of mass?
A)
1,000kg
done
clear
B)
1kg
done
clear
C)
10,000kg
done
clear
D)
100kg
done
clear
View Answer play_arrow
question_answer 41) Ethyl acetate is obtained when methyl magnesium bromide reacts with
A)
ethyl formate
done
clear
B)
ethyl chloroformate
done
clear
C)
acetyl chloride
done
clear
D)
carbon dioxide
done
clear
View Answer play_arrow
question_answer 42) The most stable hydride is
A)
\[N{{H}_{3}}\]
done
clear
B)
\[P{{H}_{3}}\]
done
clear
C)
\[As{{H}_{3}}\]
done
clear
D)
\[Sb{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 43) The ratio of amounts of\[{{H}_{2}}S\]needed to precipitate all the metal ions from 100 mL of 1 M\[AgN{{O}_{3}}\]and 100 mL of 1M\[CuS{{O}_{4}}\]will be
A)
\[1:1\]
done
clear
B)
\[1:2\]
done
clear
C)
\[2:1\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 44) If the electronegativity difference between two atoms A and B is 2.0, then the percentage of co-valent character in the molecule is
A)
54%
done
clear
B)
46%
done
clear
C)
23%
done
clear
D)
72%
done
clear
View Answer play_arrow
question_answer 45) Which of the following reaction defines\[\Delta H_{f}^{o}\]?
A)
\[{{C}_{(diamond)}}+{{O}_{2}}(g)\xrightarrow[{}]{{}}C{{O}_{2}}(g)\]
done
clear
B)
\[\frac{1}{2}{{H}_{2}}(g)+\frac{1}{2}{{F}_{2}}(g)\xrightarrow[{}]{{}}HF(g)\]
done
clear
C)
\[{{N}_{2}}(g)+3{{H}_{2}}(g)\xrightarrow[{}]{{}}2N{{H}_{3}}(g)\]
done
clear
D)
\[CO(g)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow[{}]{{}}C{{O}_{3}}(g)\]
done
clear
View Answer play_arrow
question_answer 46) Formaldehyde polymerizes to form glucose according to the reaction \[6HCHO{{C}_{6}}{{H}_{12}}{{O}_{6}}\] The theoretically computed equilibrium constant for this reaction is found to be\[6\times {{10}^{22}}\]. If 1 M solution of glucose dissociates according to the above equilibrium, the concentration of formaldehyde in the solution will be
A)
\[1.6\times {{10}^{-2}}M\]
done
clear
B)
\[1.6\times {{10}^{-4}}M\]
done
clear
C)
\[1.6\times {{10}^{-6}}M\]
done
clear
D)
\[1.6\times {{10}^{-8}}M\]
done
clear
View Answer play_arrow
question_answer 47) The electronic configuration of a dipositive ion\[{{M}^{2+}}\]is 2, 8, 14 and its mass number is 56. The number of neutrons present is
A)
32
done
clear
B)
42
done
clear
C)
30
done
clear
D)
34
done
clear
View Answer play_arrow
question_answer 48) If X is the total number of collisions which a gas molecule register with others per unit time under particular conditions, then the collision frequency of the gas containing JV molecules per unit volume is
A)
X/N
done
clear
B)
NX
done
clear
C)
2 NX
done
clear
D)
NX/2
done
clear
View Answer play_arrow
question_answer 49) A hypothetical reaction\[{{A}_{2}}+{{B}_{2}}\to 2AB\]follows the mechanism as given below, \[{{A}_{2}}\rightleftharpoons A+A\] (fast) \[A+{{B}_{2}}\xrightarrow[{}]{{}}AB+B\] (slow) \[A+B\xrightarrow[{}]{{}}AB\] (fast) The order of the overall reaction is
A)
2
done
clear
B)
1
done
clear
C)
11
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 50) The mass of helium atom of mass number 4 is 4.0026 amu, while that of the neutron and proton are 1.0087 and 1.0078 respectively on the same scale. Hence, the nuclear binding energy per nucleon in the .helium atom is nearly
A)
5MeV
done
clear
B)
7MeV
done
clear
C)
10MeV
done
clear
D)
14MeV
done
clear
View Answer play_arrow
question_answer 51) Which of the following statements is correct? Dielectric constant of\[{{H}_{2}}{{O}_{2}}\]
A)
increases with dilution
done
clear
B)
decreases with dilution
done
clear
C)
is unaffected on dilution
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 52) For the square planar complex [M] (where, M= central metal and a, b, c and d are monodentate ligands), the number of possible geometrical isomers are
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 53) Potash alum dissolves in water to give a/an
A)
acidic solution of\[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
alkaline solution
done
clear
C)
acidic solution of\[HCl\]
done
clear
D)
neutral solution
done
clear
View Answer play_arrow
question_answer 54) The discovery of which of the following group of elements gave death blow to the Newlands law of octaves?
A)
Inert gases
done
clear
B)
Alkaline earths
done
clear
C)
Rare earths
done
clear
D)
Actinides
done
clear
View Answer play_arrow
question_answer 55) Vant Hoff factor more than unity indicates that the solute in solution has
A)
dissociated
done
clear
B)
associated
done
clear
C)
Both [a] and [b]
done
clear
D)
cannot say anything
done
clear
View Answer play_arrow
question_answer 56) How many number of atoms are there in a cube based unit cell having one atom on each corner and two atoms on each body diagonal of cube?
A)
8
done
clear
B)
6
done
clear
C)
4
done
clear
D)
9
done
clear
View Answer play_arrow
question_answer 57) Bleeding due to a cut can be stopped by applying ferric chloride solution in the laboratory. This is due to
A)
co-agulation of negatively charged blood particles by\[F{{e}^{3+}}\]ions
done
clear
B)
co-agulation of positively charged blood particles by CF ions
done
clear
C)
reaction taking place between ferric ions and the haemoglobin forming a complex
done
clear
D)
common element, iron, in both\[FeC{{l}_{3}}\]and haemoglobin.
done
clear
View Answer play_arrow
question_answer 58) Which one of the following solutions will have highest conductivity?
A)
\[0.1\text{ }M\text{ }C{{H}_{3}}COOH\]
done
clear
B)
\[0.1\,M\,NaCl\]
done
clear
C)
\[0.1\,M\,KN{{O}_{3}}\]
done
clear
D)
\[0.1\,M\,HCl\]
done
clear
View Answer play_arrow
question_answer 59) One of the following metals forms a volatile compound and this property is taken advantage for its extraction. This metal is
A)
iron
done
clear
B)
nickel
done
clear
C)
cobalt
done
clear
D)
tungsten
done
clear
View Answer play_arrow
question_answer 60) If\[N{{a}^{+}}\]is larger than\[M{{g}^{2+}}\]ion an\[{{S}^{2-}}\]ion is larger than\[C{{l}^{-}}\]ion, which of the following will be stable soluble in water?
A)
Sodium chloride
done
clear
B)
Sodium sulphide
done
clear
C)
Magnesium chloride
done
clear
D)
Magnesium sulphide
done
clear
View Answer play_arrow
question_answer 61) Impurities of Cu and Ag from gold are removed by
A)
boiling impure gold with dil.\[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
boiling impure gold with cone.\[{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
electrolytically
done
clear
D)
Both [b] and [c]
done
clear
View Answer play_arrow
question_answer 62) Which of the following salt would give\[S{{O}_{2}}\] with hot and dil.\[{{H}_{2}}S{{O}_{4}}\]and also decolourises \[B{{r}_{2}}\]water ?
A)
\[N{{a}_{2}}S{{O}_{3}}\]
done
clear
B)
\[NaHS{{O}_{4}}\]
done
clear
C)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
D)
\[N{{a}_{2}}S\]
done
clear
View Answer play_arrow
question_answer 63) If two compounds have the same empirical formula but different molecular formulae, they must have
A)
different percentage composition
done
clear
B)
different molecular weights
done
clear
C)
same viscocity
done
clear
D)
same vapour density
done
clear
View Answer play_arrow
question_answer 64) Among the following which one has weakest carbon-halogen bond?
A)
Benzyl bromide
done
clear
B)
Bromobenzene
done
clear
C)
Vinyl bromide
done
clear
D)
Benzyl chloride
done
clear
View Answer play_arrow
question_answer 65) Petrochemicals can be used to prepare
A)
synthetic fibres
done
clear
B)
pesticides
done
clear
C)
plastics
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 66) tert-butyl methyl ether on heating with anhydrous HI in ether gives
A)
\[C{{H}_{3}}OH+{{(C{{H}_{3}})}_{3}}Cl\]
done
clear
B)
\[C{{H}_{3}}I+{{(C{{H}_{3}})}_{3}}COH\]
done
clear
C)
\[C{{H}_{3}}I+{{(C{{H}_{3}})}_{3}}Cl\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 67) The correctly reported answer of the addition of 4.523, 2.3 and 6.24 will have significant figures
A)
two
done
clear
B)
three
done
clear
C)
four
done
clear
D)
five
done
clear
View Answer play_arrow
question_answer 68) What happens if\[CC{{l}_{4}}\]is treated with\[AgN{{O}_{3}}\]?
A)
A white ppt. of\[AgCl\]will form
done
clear
B)
\[N{{O}_{2}}\] will be evolved
done
clear
C)
\[CC{{l}_{4}}\]will dissolve in\[AgN{{O}_{3}}\]
done
clear
D)
Nothing will happen
done
clear
View Answer play_arrow
question_answer 69) \[^{23}Na\] is more stable isotope of Na. Find out the process by which\[_{11}^{24}Na\]can undergo radioactive decay
A)
\[{{\beta }^{-}}\]emission
done
clear
B)
\[\alpha -\]emission
done
clear
C)
\[{{\beta }^{+}}\] emission
done
clear
D)
K electron capture
done
clear
View Answer play_arrow
question_answer 70) The heat of combustion of solid benzoic acid at constant volume is\[-321.30\text{ }kJ\] at\[27{}^\circ C\]. The heat of combustion at constant pressure is
A)
\[-321.30-300R\]
done
clear
B)
\[-321.30+300R\]
done
clear
C)
\[-321.30-150R\]
done
clear
D)
\[-321.30+900\text{ }R\]
done
clear
View Answer play_arrow
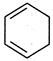
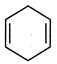
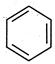
question_answer 71) In which of the following compounds\[-OH\] group is least reactive?
A)
done
clear
B)
done
clear
C)
done
clear
D)
All are equally reactive.
done
clear
View Answer play_arrow
question_answer 72) lodoform is obtained when ethanol is heated with
A)
\[KI\]and aq KOH
done
clear
B)
\[{{I}_{2}}\]and aq\[KOH\]
done
clear
C)
\[{{I}_{2}}/\]aq\[KI\]
done
clear
D)
\[HI\]and\[HI{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 73) The total number of acylic isomers including the stereoisomers (geometrical and optical), with the molecular formula\[{{C}_{4}}{{H}_{7}}Cl\]is
A)
12
done
clear
B)
11
done
clear
C)
10
done
clear
D)
9
done
clear
View Answer play_arrow
question_answer 74) The alkyl halides that can be made by free radical halogenation of alkanes are
A)
\[RCl,\]and\[RBr\]but not RP or\[RI\]
done
clear
B)
\[RF,RCl\]and\[RBr\]but not\[RI\]
done
clear
C)
\[RF,\text{ }RCl,\text{ }RBr,\text{ }RI\]
done
clear
D)
\[RF,RCl\]and\[RI\]but\[RBr\]
done
clear
View Answer play_arrow
question_answer 75) Silica is a/an
A)
acidic flux only
done
clear
B)
gangueonly
done
clear
C)
basic flux only
done
clear
D)
both gangue and acidic flux
done
clear
View Answer play_arrow
question_answer 76) The nodes present in\[3p-\]orbitals are
A)
one spherical, one planar
done
clear
B)
two spherical
done
clear
C)
two planar
done
clear
D)
one planar
done
clear
View Answer play_arrow
question_answer 77) The number of\[\alpha -\]and\[\beta -\]particles emitted in nuclear reaction\[_{90}T{{h}^{228}}{{\xrightarrow[{}]{{}}}_{83}}B{{i}^{212}}\]are respectively
A)
4, 1
done
clear
B)
3, 7
done
clear
C)
8, 1
done
clear
D)
4, 7
done
clear
View Answer play_arrow
question_answer 78) Two bottles A and B contains 1 M and 1m aqueous solution of sulphuric acid respectively
A)
A is more concentrated than B
done
clear
B)
B is more concentrated than A
done
clear
C)
concentration of A is equal to concentration of B
done
clear
D)
it is not possible to compare the concentrations
done
clear
View Answer play_arrow
question_answer 79) A salt on treatment with dil.\[HCl\] gives a pungent smelling gas and a yellow precipitate. The salt gives green flame test and a yellow precipitate with potassium chromate the salt is
A)
\[NiS{{O}_{4}}\]
done
clear
B)
\[Ba{{S}_{2}}{{O}_{3}}\]
done
clear
C)
\[Pb{{S}_{2}}{{O}_{3}}\]
done
clear
D)
\[CuS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 80) Which of the oxide of manganese is amphoteric?
A)
\[Mn{{O}_{2}}\]
done
clear
B)
\[M{{n}_{2}}{{O}_{3}}\]
done
clear
C)
\[M{{n}_{2}}{{O}_{7}}\]
done
clear
D)
\[MnO\]
done
clear
View Answer play_arrow
question_answer 81) Which of the following alkenes is most reactive towards cationic polymerization?
A)
\[C{{H}_{2}}=CHC{{H}_{3}}\]
done
clear
B)
\[{{H}_{2}}C=CHCl\]
done
clear
C)
\[{{H}_{2}}C=CH{{C}_{6}}{{H}_{5}}\]
done
clear
D)
\[{{H}_{2}}C=CHC{{O}_{2}}C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 82) An organic compound,\[{{C}_{3}}{{H}_{6}}O\]does not give a precipitate with 2, 4-dinitrophenyl hydrazine reagent and does not react with metallic sodium. It could be
A)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
B)
\[C{{H}_{2}}=CHC{{H}_{2}}OH\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{2}}=CHOC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 83) Oxidation of 1-butene with hot\[KMn{{O}_{4}}\]solution produces
A)
\[C{{H}_{3}}C{{H}_{2}}COOH+HCOOH\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}COOH+C{{O}_{2}}\]
done
clear
C)
\[C{{H}_{3}}COOH+C{{O}_{2}}\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}C=O+C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 84) A mixture of 1-chlorobutane and 2-chloro- butane when treated with alcoholic KOH gives:
A)
1-butene
done
clear
B)
2-butene
done
clear
C)
isobutylene
done
clear
D)
mixture of 1-butene +2-butene
done
clear
View Answer play_arrow
question_answer 85)
Out of the two compounds shown below, the vapour pressure of B at a particular temperature is expected to be
A)
higher than that of A
done
clear
B)
lower than that of B
done
clear
C)
same as that of A
done
clear
D)
can be higher or lower depending upon the size of the vessel.
done
clear
View Answer play_arrow
question_answer 86) Roasted tin stone ore after washing with water is known as
A)
block tin
done
clear
B)
white tin
done
clear
C)
black tin
done
clear
D)
granulated tin
done
clear
View Answer play_arrow
question_answer 87) Which of the following has strongest hydrogen bonding?
A)
Ethylamine
done
clear
B)
Ammonia
done
clear
C)
Ethyl alcohol
done
clear
D)
Diethyl ether
done
clear
View Answer play_arrow
question_answer 88) Consider the following statements The rate law for the acid catalysed hydrolysis of an ester being given as Rate\[=k[{{H}^{+}}]\] [ester]\[=k\][ester]. If the acid concentration is doubled at constant ester concentration 1. The second order rate constant, k is doubled. 2. The pseudo first order rate constant, k is doubled. 3. The rate of the reaction is doubled Which of the above statements are correct?
A)
1 and 2
done
clear
B)
2 and 3
done
clear
C)
1 and 3
done
clear
D)
1, 2 and 3
done
clear
View Answer play_arrow
question_answer 89) A fibrous mineral which can withstand red hot flames without any damage is
A)
talc
done
clear
B)
glass wool
done
clear
C)
soap stone
done
clear
D)
asbestos
done
clear
View Answer play_arrow
question_answer 90) When o- or\[p-\]phenolsulphonic acid is treated with bromine water, the product formed is
A)
2, 4-dibromophenol
done
clear
B)
2, 4, 6-tribromophenol
done
clear
C)
3-bromophenol boric acid
done
clear
D)
3, 5-dibromophenol
done
clear
View Answer play_arrow
question_answer 91) An alkene on vigorous oxidation with\[KMn{{O}_{4}}\]gives only acetic acid. The alkene is
A)
\[C{{H}_{3}}C{{H}_{2}}CH=C{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}CH=CHC{{H}_{3}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}C=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}CH=C{{H}_{2}}\]
done
clear
View Answer play_arrow
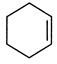
question_answer 92) Which of the following has the maximum heat of hydrogenation?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 93) Deflection back of a few particles on hitting thin foil of gold shows that
A)
nucleus is heavy
done
clear
B)
nucleus is small
done
clear
C)
Both [a] and [b]
done
clear
D)
electrons create hinderance in the movement of\[\alpha -\]particles
done
clear
View Answer play_arrow
question_answer 94) Which of the following does not form an oxime?
A)
Glucose
done
clear
B)
Glucose penta-acetate
done
clear
C)
Arabinose
done
clear
D)
Galactose
done
clear
View Answer play_arrow
question_answer 95) An aromatic compounds X with molecular formul\[{{C}_{8}}{{H}_{10}}\]produces on nitration one mononitro derivative and three dinitro derivatives. Compound X would be
A)
ethyl benzene
done
clear
B)
\[m-\]xylene
done
clear
C)
\[o-\]xylene
done
clear
D)
\[p-\]xylene
done
clear
View Answer play_arrow
question_answer 96) The end product C in the following sequence of chemical reactions is \[C{{H}_{3}}COOH\xrightarrow[{}]{CaC{{O}_{3}}}A\xrightarrow[{}]{heat}B\xrightarrow[{}]{N{{H}_{2}}OH}C\]
A)
acetaldehyde oxime
done
clear
B)
formaldehyde oxime
done
clear
C)
methyl nitrate
done
clear
D)
acetoxime
done
clear
View Answer play_arrow
question_answer 97) The only cations present in a slightly acidic solution are\[F{{e}^{3+}},Z{{n}^{2+}}\]and\[C{{u}^{2+}}\]The reagent that when added in excess to this solution would identify and separate\[F{{e}^{3+}}\] in one step is
A)
\[2M\,HCl\]
done
clear
B)
\[6M\,N{{H}_{3}}\]
done
clear
C)
\[6M\,NaOH\]
done
clear
D)
\[{{H}_{2}}S\,gas\]
done
clear
View Answer play_arrow
question_answer 98) The nucleus of an atom can he assumed to be spherical. The radius of the nucleus of mass number A is given by\[1.25\times {{10}^{-13}}\times {{A}^{1/3}}cm\]. Radius of atom is one A. If the mass number is 64, then the fraction of the atomic volume that is occupied by the nucleus is
A)
\[1.0\times {{10}^{-3}}\]
done
clear
B)
\[5.0\times {{10}^{-5}}\]
done
clear
C)
\[2.5\times {{10}^{-2}}\]
done
clear
D)
\[1.25\times {{10}^{-13}}\]
done
clear
View Answer play_arrow
question_answer 99) Carboxylic acids readily dissolve in aqueous sodium bicarbonate, liberating carbon dioxide. Which one of the following is correct?
A)
Free carboxylic acid and its conjugate base are of comparable stability
done
clear
B)
The free carboxylic acid is more stable than its conjugate base
done
clear
C)
The conjugate base of the carboxylic acid is more stable than the free carboxylic acid
done
clear
D)
The conjugate acid of the carboxylic acid is more stable than the free carboxylic acid
done
clear
View Answer play_arrow
question_answer 100) Lead pipes can be used safely for carrying
A)
soft water
done
clear
B)
soft water first heated with lime stone
done
clear
C)
conc. \[HN{{O}_{3}}\]
done
clear
D)
soft as well as hard water
done
clear
View Answer play_arrow
question_answer 101) The branch of biology, which deals with the study of processes and functions of an organism, is called
A)
histology
done
clear
B)
anatomy
done
clear
C)
physiology
done
clear
D)
entomology
done
clear
View Answer play_arrow
question_answer 102) Virus multiplies in
A)
soil
done
clear
B)
dead tissue
done
clear
C)
living tissue
done
clear
D)
culture medium
done
clear
View Answer play_arrow
question_answer 103) Which of the following technique, other than microscopy is used for study of cell ?
A)
Maceration
done
clear
B)
Plasmolysis
done
clear
C)
Chromatography
done
clear
D)
Autoradiography
done
clear
View Answer play_arrow
question_answer 104) Robert Hooke used the term cell in the year
A)
1650
done
clear
B)
1665
done
clear
C)
1865
done
clear
D)
1960
done
clear
View Answer play_arrow
question_answer 105) Protein synthesis takes place in
A)
ribosomes
done
clear
B)
chloroplasts
done
clear
C)
mitochondria
done
clear
D)
Golgi bodies
done
clear
View Answer play_arrow
question_answer 106) Replication of centriole occurs during
A)
interphase
done
clear
B)
prophase
done
clear
C)
late prophase
done
clear
D)
late telophase
done
clear
View Answer play_arrow
question_answer 107) L-shaped chromosomes are also called
A)
acrocentric
done
clear
B)
telocentric
done
clear
C)
sub-metacentric
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 108) Which of the following is/are grouped under phanerogams?
A)
Angiosperms
done
clear
B)
Gymnosperms
done
clear
C)
Pteridophytes
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 109) A bacterium divides after every 35 minutes. If a culture containing \[{{10}^{5}}\]cells per mL is grown, then cell concentration per mL after 175 minutes will be
A)
\[175\times {{10}^{5}}\]
done
clear
B)
\[125\times {{10}^{5}}\]
done
clear
C)
\[48\times {{10}^{5}}\]
done
clear
D)
\[32\times {{10}^{5}}\]
done
clear
View Answer play_arrow
question_answer 110) Cladonia rangiferina is a/an
A)
algae
done
clear
B)
lichen
done
clear
C)
fungus
done
clear
D)
angiosperm
done
clear
View Answer play_arrow
question_answer 111) Which of the following is an algal parasite?
A)
Volvox
done
clear
B)
Ulothrix
done
clear
C)
Porphyra
done
clear
D)
Cephaleuros
done
clear
View Answer play_arrow
question_answer 112) Which of the following is true about bryophytes ?
A)
They are thalloid
done
clear
B)
They contain chloroplast
done
clear
C)
They possess archegonia
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 113) The kidney-shaped covering of sorus Dryopteris, is called
A)
placenta
done
clear
B)
ramentum
done
clear
C)
sporophyll
done
clear
D)
indusium
done
clear
View Answer play_arrow
question_answer 114) Which of the following is a wheat fruit?
A)
Achene
done
clear
B)
Cypsella
done
clear
C)
Caryopsis
done
clear
D)
Endosperm
done
clear
View Answer play_arrow
question_answer 115) A gymnospermic leaf carries 16 chromosomes. The number of chromosomes in its endosperm is
A)
24
done
clear
B)
16
done
clear
C)
12
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 116) Tyioses thickenings are seen in
A)
collenchyma
done
clear
B)
phloem cells
done
clear
C)
ray parenchyma only
done
clear
D)
ray parenchyma and xylem cells
done
clear
View Answer play_arrow
question_answer 117) Which of the following plant shows multiple epidermis?
A)
Croton
done
clear
B)
Allium
done
clear
C)
Nerium
done
clear
D)
Cucurbita
done
clear
View Answer play_arrow
question_answer 118) Double fertilization occurs among
A)
algae
done
clear
B)
bryophytes
done
clear
C)
angiosperms
done
clear
D)
gymnospenr
done
clear
View Answer play_arrow
question_answer 119) The endosperm in angiosperm develops from
A)
zygote
done
clear
B)
secondary nucleus
done
clear
C)
chalazal polar nucleus
done
clear
D)
micropylar polar nucleus
done
clear
View Answer play_arrow
question_answer 120) The fertilization in which male gametes are carried through pollen tube is known as
A)
syngamy
done
clear
B)
porogamy
done
clear
C)
siphonogamy
done
clear
D)
chalazogamy
done
clear
View Answer play_arrow
question_answer 121) Which of the following theory gives the latest explanation for the closure of stomata ?
A)
ABA theory
done
clear
B)
Munch theory
done
clear
C)
Starch glucose theory
done
clear
D)
Active \[{{K}^{+}}\]transport theory
done
clear
View Answer play_arrow
question_answer 122) Loss of liquid water by guttation occurs through
A)
hydathodes
done
clear
B)
stomata
done
clear
C)
cuticle
done
clear
D)
bark
done
clear
View Answer play_arrow
question_answer 123) Which of the following is the first compound that accepts carbon dioxide during dark phase of photosynthesis?
A)
NADP
done
clear
B)
RuBP
done
clear
C)
Ferredoxin
done
clear
D)
Cytochrome
done
clear
View Answer play_arrow
question_answer 124) Oxygen which is liberated during photosynthesis, comes from
A)
\[C{{O}_{2}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
chlorophyll
done
clear
D)
phosphoglyceric acid
done
clear
View Answer play_arrow
question_answer 125) In \[{{C}_{4}}\] plants, the carbon dioxide fixation occurs in
A)
guard cells
done
clear
B)
spongy cells
done
clear
C)
palisade cells
done
clear
D)
bundle sheath cells
done
clear
View Answer play_arrow
question_answer 126) Ethyl alcohol is commercially manufactured from
A)
bajra
done
clear
B)
grapes
done
clear
C)
maize
done
clear
D)
sugarcane
done
clear
View Answer play_arrow
question_answer 127) Last electron acceptor in respiration is
A)
\[{{O}_{2}}\]
done
clear
B)
\[{{H}_{2}}\]
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
NADH
done
clear
View Answer play_arrow
question_answer 128) A hormone delaying senescence is
A)
auxin
done
clear
B)
cytokinins
done
clear
C)
ethylene
done
clear
D)
gibberellin
done
clear
View Answer play_arrow
question_answer 129) Which of the following induces flowering in long day plants ?
A)
Gibberellins
done
clear
B)
Cytokinin
done
clear
C)
Auxins
done
clear
D)
Ethylene
done
clear
View Answer play_arrow
question_answer 130) What name has been assigned to the genus produced by a cross between cabbage and radish?
A)
Secale
done
clear
B)
Bursapastoris
done
clear
C)
Lysogenicophyll
done
clear
D)
Raphanobrassica
done
clear
View Answer play_arrow
question_answer 131) Genetic counsellors can identify heterozygous individuals by
A)
height of individuals
done
clear
B)
colour of individuals
done
clear
C)
screening procedures
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 132) The term heterosis was first coined by
A)
McClintock
done
clear
B)
Poweri
done
clear
C)
Swaminathan
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 133) Somatic hybridization is a technique of
A)
natural breeding
done
clear
B)
natural pollination
done
clear
C)
artificial pollination
done
clear
D)
somatic cells hybridization
done
clear
View Answer play_arrow
question_answer 134) which of the following is initiation codon in eukaryotes ?
A)
UAG
done
clear
B)
UGA
done
clear
C)
UAA
done
clear
D)
AUG
done
clear
View Answer play_arrow
question_answer 135) Which of the following species are restricted to an area?
A)
Sibling species
done
clear
B)
Endemic species
done
clear
C)
Allopatric species
done
clear
D)
Sympatric species
done
clear
View Answer play_arrow
question_answer 136) Biological concept of species is mainly based on
A)
reproductive isolation
done
clear
B)
morphological features only
done
clear
C)
methods of reproduction only
done
clear
D)
morphology and methods of reproduction
done
clear
View Answer play_arrow
question_answer 137) Which of the following supports a dense population of plankton and littoral vegetation?
A)
Oligotrophic
done
clear
B)
Eutrophic
done
clear
C)
Lithotrophic
done
clear
D)
Agroecotrophic
done
clear
View Answer play_arrow
question_answer 138) A man-made ecosystem is
A)
less in diversity
done
clear
B)
more in diversity
done
clear
C)
man does not make ecosystem
done
clear
D)
more stable than natural ecosystem
done
clear
View Answer play_arrow
question_answer 139) Crop rotation is used by farmers to increase
A)
soil fertility
done
clear
B)
community area
done
clear
C)
organic content of soil
done
clear
D)
nitrogenous content in the soil
done
clear
View Answer play_arrow
question_answer 140) Which of the following organism form the decomposers ?
A)
Pteris
done
clear
B)
Bacteria
done
clear
C)
Saprophytic fungi
done
clear
D)
Both [b] and [c]
done
clear
View Answer play_arrow
question_answer 141) Fly-ash is a/an
A)
insectivorous plant
done
clear
B)
light airborn paniculate matter
done
clear
C)
new name of orchid plant
done
clear
D)
causal organism of various diseases
done
clear
View Answer play_arrow
question_answer 142) Some effects of sulphur dioxide and its transformation products on plant include
A)
plasmolysis
done
clear
B)
Golgi body destruction
done
clear
C)
protein dis-integration
done
clear
D)
chlorophyll destruction
done
clear
View Answer play_arrow
question_answer 143) Which of the following plant is used for the purification of water?
A)
Beggiata
done
clear
B)
Chlorella
done
clear
C)
Spirogyra
done
clear
D)
Eichhornia
done
clear
View Answer play_arrow
question_answer 144) In the treatment of waste water discharge, which treatment stage involves biological treatment?
A)
Primary treatment
done
clear
B)
Secondaiy treatment
done
clear
C)
Tertiary treatment
done
clear
D)
Reverse osmosis stage
done
clear
View Answer play_arrow
question_answer 145) Which of the following is considered to be the best chemical method of fixing atmospheric nitrogen?
A)
Fisher method
done
clear
B)
Decan method
done
clear
C)
Haber-Bosch method
done
clear
D)
Paranas-Meyerhoff method
done
clear
View Answer play_arrow
question_answer 146) Desired improved varieties of economically useful crops are raised by
A)
migration
done
clear
B)
biofertilizer
done
clear
C)
hybridization
done
clear
D)
natural selection
done
clear
View Answer play_arrow
question_answer 147) Indian rose wood tree is a common name of
A)
Acacia
done
clear
B)
Shorea
done
clear
C)
Dalbergia
done
clear
D)
Eucalyptus
done
clear
View Answer play_arrow
question_answer 148) Emasculation is concerned with
A)
hybridization
done
clear
B)
clonal selection
done
clear
C)
mass selection
done
clear
D)
pure line selection
done
clear
View Answer play_arrow
question_answer 149) Which of the following insecticide is obtained from the roots of Derris elleptica ?
A)
Cinerin
done
clear
B)
Nicotine
done
clear
C)
Rotenone
done
clear
D)
Pyrethrum
done
clear
View Answer play_arrow
question_answer 150) In maize, hybrid vigour is exploited by
A)
bombarding the protoplast with DNA
done
clear
B)
crossing of two inbreed parental lines
done
clear
C)
harvesting seeds from the most productive plants
done
clear
D)
inducing mutations
done
clear
View Answer play_arrow
question_answer 151) Which of the following is not vestigial in man?
A)
Tail vertebrae
done
clear
B)
Nails
done
clear
C)
Nictitating membrane
done
clear
D)
Vermiform appendix
done
clear
View Answer play_arrow
question_answer 152) The chemical used in National Malaria Eradication Programme is
A)
2,4-D
done
clear
B)
BHC
done
clear
C)
DDT
done
clear
D)
Pyrethroid
done
clear
View Answer play_arrow
question_answer 153) A eukaryotic gene contains two kinds of base sequences. Which of these plays an important role in protein synthesis ?
A)
Introns
done
clear
B)
Exons
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 154) The number of hydrogen bonds between adenine and thymine in a DNA molecule is
A)
two
done
clear
B)
three
done
clear
C)
four
done
clear
D)
eight
done
clear
View Answer play_arrow
question_answer 155) The enzyme, which combines with non-protein part to form a functional enzyme known as
A)
Co-enzyme
done
clear
B)
holoenzyme
done
clear
C)
apoenzyme
done
clear
D)
prosthetic group
done
clear
View Answer play_arrow
question_answer 156) Which of the following enzyme digest protein in stomach ?
A)
Trypsin
done
clear
B)
Pepsin
done
clear
C)
Crepsin
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 157) Passive food ingestion in Amoeba is known as
A)
import
done
clear
B)
invagination
done
clear
C)
circumfluence
done
clear
D)
circumvallatic-
done
clear
View Answer play_arrow
question_answer 158) The slime moulds are characterized by the presence of
A)
elaters
done
clear
B)
pseudoelaters
done
clear
C)
capillitium
done
clear
D)
capitulum
done
clear
View Answer play_arrow
question_answer 159) Ecdysone is secreted from
A)
Insecta
done
clear
B)
Trematoda
done
clear
C)
Nematoda
done
clear
D)
Polychaeta
done
clear
View Answer play_arrow
question_answer 160) In the life cycle of mosquito, comma-shaped stage is
A)
larval stage
done
clear
B)
pupal stage
done
clear
C)
imago stage
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 161) Haemocoel is found in
A)
Hydra and Aurelia
done
clear
B)
Taenia and Ascaris
done
clear
C)
Cockroach and Pila
done
clear
D)
Balanoglossus and Herdmania
done
clear
View Answer play_arrow
question_answer 162) The group of Anamniota includes
A)
reptiles and birds
done
clear
B)
birds and mammals
done
clear
C)
fishes and amphibians
done
clear
D)
reptiles and mammals
done
clear
View Answer play_arrow
question_answer 163) The excretory material of bony fish is
A)
urea
done
clear
B)
protein
done
clear
C)
ammonia
done
clear
D)
amino acid
done
clear
View Answer play_arrow
question_answer 164) Different colour of frog skin are controller by
A)
hormones
done
clear
B)
melanocytes
done
clear
C)
nervous system
done
clear
D)
Both [a] and [c]
done
clear
View Answer play_arrow
question_answer 165) Blastula of frog has
A)
blastopore
done
clear
B)
blastocoel
done
clear
C)
archenteron
done
clear
D)
gastropore
done
clear
View Answer play_arrow
question_answer 166) Carotene pigment is found in the cells of
A)
dermis
done
clear
B)
epidermis
done
clear
C)
adipose cell
done
clear
D)
Both [b] and [c]
done
clear
View Answer play_arrow
question_answer 167) Deboves membrane is a layer of
A)
muscular tissue
done
clear
B)
epithelial tissue
done
clear
C)
connective tissue
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 168) Achilles tendon is associated with
A)
gluteus muscle
done
clear
B)
hamstring muscle
done
clear
C)
quadriceps muscle
done
clear
D)
gastrocnemius muscle
done
clear
View Answer play_arrow
question_answer 169) The leucocytes contain, which of the following in large quantity?
A)
Basophils
done
clear
B)
Neutrophils
done
clear
C)
Eosinophils
done
clear
D)
Monocytes
done
clear
View Answer play_arrow
question_answer 170) Which part of our body secreted the hormone secretin ?
A)
Ileum
done
clear
B)
Stomach
done
clear
C)
Duodenum
done
clear
D)
Oesophagus
done
clear
View Answer play_arrow
question_answer 171) During inspiration, the diaphragm
A)
expands
done
clear
B)
shows no change
done
clear
C)
contracts and flattens
done
clear
D)
relaxes to become dome-shaped
done
clear
View Answer play_arrow
question_answer 172) The oxygen toxicity is related with
A)
blood poisoning
done
clear
B)
collapse of alveolar walls
done
clear
C)
failure of ventilation of lungs
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 173) Cardiac output is determined by
A)
heart rate
done
clear
B)
stroke volume
done
clear
C)
blood flow
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 174) The important function of lymph is to
A)
transport oxygen to the brain
done
clear
B)
transport carbon dioxide to the lungs
done
clear
C)
return RBCs to the lymph nodes
done
clear
D)
return interstitial fluid to the blood
done
clear
View Answer play_arrow
question_answer 175) The lining of intestine and kidneys in humans is
A)
keratinized
done
clear
B)
brush border
done
clear
C)
ciliated
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 176) The yellow colour of urine is due to the presence of
A)
urea
done
clear
B)
uric acid
done
clear
C)
urochrome
done
clear
D)
bilirubin
done
clear
View Answer play_arrow
question_answer 177) The Leydig cells secrete
A)
oestrogen
done
clear
B)
testosterone
done
clear
C)
progesterone
done
clear
D)
corticosterone
done
clear
View Answer play_arrow
question_answer 178) The function of pineal body is to
A)
lighten the skin colours
done
clear
B)
control sexual behaviour
done
clear
C)
regulates the period of puberty
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 179) Which of the following nerve is purely motor nerve ?
A)
Vagus
done
clear
B)
Facial
done
clear
C)
Abducens
done
clear
D)
Trigeminal
done
clear
View Answer play_arrow
question_answer 180) Which of the following part of a neuron is covered by fatty sheath?
A)
Axon
done
clear
B)
Cyton
done
clear
C)
Dendrite
done
clear
D)
Node of Ranvier
done
clear
View Answer play_arrow
question_answer 181) Synsacrum of fowl is consist of about
A)
29 vertebrae
done
clear
B)
3 vertebrae
done
clear
C)
16 vertebrae
done
clear
D)
single vertebrae
done
clear
View Answer play_arrow
question_answer 182) In rabbit, head of the epididymis present at the head of the testes, is called
A)
vas deferens
done
clear
B)
gubemaculum
done
clear
C)
cauda epididymis
done
clear
D)
caput epididymis
done
clear
View Answer play_arrow
question_answer 183) The extra embryonic membranes of mammalian embryo are derived from
A)
trophoblast
done
clear
B)
follicle cells
done
clear
C)
formative cells
done
clear
D)
inner cell mass
done
clear
View Answer play_arrow
question_answer 184) The eggs of silk moth are
A)
homolecithal
done
clear
B)
telolecithal
done
clear
C)
mesolecithal
done
clear
D)
centrolecithal
done
clear
View Answer play_arrow
question_answer 185) Which of the following was formed in S. Millers experiment?
A)
Amino acids
done
clear
B)
Nucleic acids
done
clear
C)
UV radiations
done
clear
D)
Microspheres
done
clear
View Answer play_arrow
question_answer 186) Trilobites were evolved during, which of the following period?
A)
Silurian
done
clear
B)
Cambrian
done
clear
C)
Ordovician
done
clear
D)
Precambrian
done
clear
View Answer play_arrow
question_answer 187) Which of the following variations are temporary and have nothing to do with the last or next generation?
A)
Hereditary variations
done
clear
B)
Discontinuous variations
done
clear
C)
Environmental variations
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 188) The modem man differs from the apes in
A)
protruding eyes
done
clear
B)
spare body hair
done
clear
C)
wearing of clothes
done
clear
D)
arms shorter than legs
done
clear
View Answer play_arrow
question_answer 189) The highest cranial capacity is/was present in
A)
Java man
done
clear
B)
peking man
done
clear
C)
handy man
done
clear
D)
modem man
done
clear
View Answer play_arrow
question_answer 190) Wild life is
A)
any living organism in any habitat
done
clear
B)
predatory animals in their natural habitat
done
clear
C)
any living organisms in its natural habitat
done
clear
D)
economically important animals and plants
done
clear
View Answer play_arrow
question_answer 191) A marriage between normal visioned man and colourblind woman will produce, which of the following type of offsprings ?
A)
Normal sons and carrier daughters
done
clear
B)
Colourblind sons and carrier daughters
done
clear
C)
Colourblind sons and 50% carrier daughters
done
clear
D)
50% colourblind sons and 50% carrier daughters
done
clear
View Answer play_arrow
question_answer 192) Haemophilia is a
A)
deficiency disorder
done
clear
B)
Y-linked disorder
done
clear
C)
X-linked disorder
done
clear
D)
autosomal sex disorder
done
clear
View Answer play_arrow
question_answer 193) UV radiation from sun causes, which of the following disorder of eyes ?
A)
Cataract
done
clear
B)
Glaucoma
done
clear
C)
Dilation pupil
done
clear
D)
Some defect of retina
done
clear
View Answer play_arrow
question_answer 194) The study of relationship between size and shape is called
A)
allopatric
done
clear
B)
allelopathy
done
clear
C)
allometry
done
clear
D)
allogamy
done
clear
View Answer play_arrow
question_answer 195) Which of the following is the most sparsely populated state of India ?
A)
Manipur
done
clear
B)
Rajasthan
done
clear
C)
Meghalaya
done
clear
D)
Arunachal Pradesh
done
clear
View Answer play_arrow
question_answer 196) The natural parthenogenesis is found in
A)
sharks
done
clear
B)
housefly
done
clear
C)
Drosophilia
done
clear
D)
honey bee
done
clear
View Answer play_arrow
question_answer 197) The interferons are
A)
antiviral proteins
done
clear
B)
antibiotic proteins
done
clear
C)
antigen proteins
done
clear
D)
antibacterial partied
done
clear
View Answer play_arrow
question_answer 198) An organic substance bound to an enzyme and essential for its activity is called
A)
Co-enzyme
done
clear
B)
holoenzyme
done
clear
C)
apoenzyme
done
clear
D)
isoenzyme
done
clear
View Answer play_arrow
question_answer 199) Who coined the term antibiotics?
A)
Darwin
done
clear
B)
Woodruff
done
clear
C)
Flemming
done
clear
D)
Selman Waksman
done
clear
View Answer play_arrow
question_answer 200) A complex polysaccharide produced from sucrose by the bacterium Leuconostoc mesenteries is
A)
chitin
done
clear
B)
starch
done
clear
C)
cellulose
done
clear
D)
dextran
done
clear
View Answer play_arrow