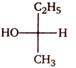
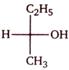
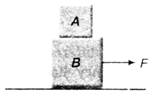
question_answer 1)
Block A of mass 4 kg and block B of mass 6 kg are resting on a horizontal surface as shown in the figure. There is no friction between the block B and the horizontal surface. The coefficient of friction between the blocks is 0.2. If the value of \[g=10\,m/{{s}^{2}},\] the maximum horizontal force F that can be applied on block B without any relative motion between A and B is
A)
20 N
done
clear
B)
40 N
done
clear
C)
60 N
done
clear
D)
100 N
done
clear
View Answer play_arrow
question_answer 2) A body is rotating with angular momentum L. If \[I\]is its moment of inertia about the axis of rotation, its kinetic energy of rotation is
A)
\[\frac{1}{2}I{{L}^{2}}\]
done
clear
B)
\[\frac{1}{2}IL\]
done
clear
C)
\[\frac{1}{2}{{I}^{2}}L\]
done
clear
D)
\[\frac{1}{2}\frac{{{L}^{2}}}{I}\]
done
clear
View Answer play_arrow
question_answer 3) The unit of surface tension is
A)
N-m
done
clear
B)
N/m
done
clear
C)
\[N-{{m}^{2}}\]
done
clear
D)
N
done
clear
View Answer play_arrow
question_answer 4) The velocity of water flowing in a non-uniform tube is 20 cm/s at a point where the tube radius is 0.2 cm. The velocity at another point, where the radius is 0.1 cm, is
A)
80 cm/s
done
clear
B)
40 cm/s
done
clear
C)
20 cm/s
done
clear
D)
5 cm/s
done
clear
View Answer play_arrow
question_answer 5) The excess pressure inside a soap bubble A is twice that in another soap bubble B. The ratio of volumes of A and B is
A)
1 : 2
done
clear
B)
1 : 4
done
clear
C)
1 : 8
done
clear
D)
1 : 16
done
clear
View Answer play_arrow
question_answer 6) A body at rest splits into three parts of mass m, m and 4m respectively. The two equal masses fly off perpendicular to each other and each with speed of v. The speed of 4m will be
A)
\[\frac{v}{2\sqrt{2}}\]
done
clear
B)
\[\frac{v}{\sqrt{2}}\]
done
clear
C)
\[\frac{v}{2}\]
done
clear
D)
\[\sqrt{2\,v}\]
done
clear
View Answer play_arrow
question_answer 7) 300 cal of heat is supplied to raise the temperature of 50 g of air from \[20{}^\circ C\] to \[30{}^\circ C\] without any change in its volume. Change in internal energy per gram of air is
A)
zero
done
clear
B)
0.6 cal
done
clear
C)
1.2 cal
done
clear
D)
6.0 cal
done
clear
View Answer play_arrow
question_answer 8) The thermal capacity of any body is
A)
a measure of its capacity to absorb heat
done
clear
B)
a measure of its capacity to provide heat
done
clear
C)
the quantity of heat required to raise its temperature by a unit degree
done
clear
D)
the quantity of heat required to raise the temperature of a unit mass of the body by a unit degree
done
clear
View Answer play_arrow
question_answer 9) The absorptive power of a perfectly black body is
A)
zero
done
clear
B)
infinite
done
clear
C)
0.5
done
clear
D)
1.0
done
clear
View Answer play_arrow
question_answer 10) A diatomic molecule has
A)
1 degree of freedom
done
clear
B)
3 degrees of freedom
done
clear
C)
5 degrees of freedom
done
clear
D)
6 degrees of freedom
done
clear
View Answer play_arrow
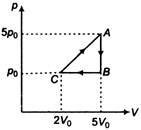
question_answer 11)
The work done by a gas taken through the closed process ABCA (see figure) is
A)
\[6{{P}_{0}}{{V}_{0}}\]
done
clear
B)
\[4{{P}_{0}}{{V}_{0}}\]
done
clear
C)
\[{{P}_{0}}{{V}_{0}}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 12) The displacement x as a function of time t of a simple harmonic motion is represented by
A)
\[\frac{{{d}^{2}}x}{d{{t}^{2}}}-{{A}^{2}}x=0\]
done
clear
B)
\[\frac{dx}{dt}+{{A}^{2}}x=0\]
done
clear
C)
\[\frac{{{d}^{2}}x}{dt}+{{A}^{2}}{{x}^{2}}=0\]
done
clear
D)
\[\frac{{{d}^{2}}x}{d{{t}^{2}}}+{{A}^{2}}x=0\]
done
clear
View Answer play_arrow
question_answer 13) The potential energy of spring when stretched by a distance x is E. The energy of the spring when stretched by x/2 is
A)
E
done
clear
B)
E/2
done
clear
C)
E/4
done
clear
D)
E/6
done
clear
View Answer play_arrow
question_answer 14) The air column in a pipe with both ends open vibrates with a fundamental frequency \[f.\] If one of the ends of the pipe is closed, the fundamental frequency will be
A)
\[f\]
done
clear
B)
\[2f\]
done
clear
C)
(0) \[\frac{3}{2}f\]
done
clear
D)
\[\frac{f}{2}\]
done
clear
View Answer play_arrow
question_answer 15) The displacement \[y\] of a particle varies with time t, in second, as \[y=2\,\cos (\pi t+\pi /6)\]. The time period of the oscillations is
A)
2s
done
clear
B)
4s
done
clear
C)
1s
done
clear
D)
0.5s
done
clear
View Answer play_arrow
question_answer 16) The number of beats per second resulting from the vibration\[{{x}_{1}}=a\,\cos \,500\,\pi t\] and\[{{x}_{2}}=a\,\cos \,508\,\pi t\] is
A)
zero
done
clear
B)
2
done
clear
C)
4
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 17) A point object is placed at the focus of a double concave lens. The image is formed
A)
at infinity
done
clear
B)
between the focus and the lens
done
clear
C)
at focus
done
clear
D)
between the focus and infinity
done
clear
View Answer play_arrow
question_answer 18) A ray of light travelling in air is incident on a medium of refractive index\[\mu .\] If the angle of refraction is twice the incident angle, the incident angle is
A)
\[\sin {{\,}^{-1}}\left( \frac{1}{2\mu } \right)\]
done
clear
B)
\[\sin {{\,}^{-1}}(2\mu )\]
done
clear
C)
\[\cos {{\,}^{-1}}(2\mu )\]
done
clear
D)
\[\cos {{\,}^{-1}}\left( \frac{1}{2\mu } \right)\]
done
clear
View Answer play_arrow
question_answer 19) Two coherent light sources emit light of the
A)
same intensity
done
clear
B)
same pitch
done
clear
C)
constant but different wavelengths
done
clear
D)
same frequency having constant phase difference
done
clear
View Answer play_arrow
question_answer 20) If in Fraunhofer diffraction due to a single slit, the slit width is increased, then the width .of the central maximum will
A)
increase
done
clear
B)
decrease
done
clear
C)
not change
done
clear
D)
change depending upon the wavelength of light used
done
clear
View Answer play_arrow
question_answer 21) The ratio of the coulomb to the gravitational force between two electrons is of the order of
A)
\[{{10}^{55}}\]
done
clear
B)
\[{{10}^{42}}\]
done
clear
C)
\[{{10}^{28}}\]
done
clear
D)
\[{{10}^{12}}\]
done
clear
View Answer play_arrow
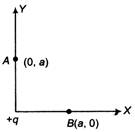
question_answer 22)
Work done in moving a charge Q from the point B(x = a, y = 0) to the point A(x = 0, y = a) in the field of charge +q at origin is
A)
zero
done
clear
B)
\[\frac{qQ}{4\pi {{\varepsilon }_{0}}(\sqrt{2}a)}\]
done
clear
C)
\[\frac{qQ}{4\pi {{\varepsilon }_{0}}(2a)}\]
done
clear
D)
\[\frac{qQ\sqrt{2}a}{4\pi {{\varepsilon }_{0}}}\]
done
clear
View Answer play_arrow
question_answer 23) A charge +Q is placed at the centre of a spherical surface of radius R. Another charge -Q/2 is placed at a distance of R/2 from the centre of the surface. A third charge -Q/2 is at a distance 3R/2 from the centre. The electrostatic flux linked with the surface is
A)
zero
done
clear
B)
\[\frac{Q}{2{{\varepsilon }_{0}}}\]
done
clear
C)
\[\frac{3Q}{2{{\varepsilon }_{0}}}\]
done
clear
D)
\[\frac{2Q}{{{\varepsilon }_{0}}}\]
done
clear
View Answer play_arrow
question_answer 24) The uniform electric field in the space between the plates of a parallel plate capacitor of plate separation d and plate areas A is E. The energy of this charged capacitor is
A)
\[\frac{1}{2}\frac{{{\varepsilon }_{0}}{{E}^{2}}}{Ad}\]
done
clear
B)
\[{{\varepsilon }_{0}}{{E}^{2}}Ad\]
done
clear
C)
\[\frac{1}{2}{{\varepsilon }_{0}}{{E}^{2}}Ad\]
done
clear
D)
\[\frac{1}{2}\frac{{{\varepsilon }_{0}}{{E}^{2}}}{Ad}\]
done
clear
View Answer play_arrow
question_answer 25) The capacity of a parallel plate capacitor without any dielectric is C. If the distance between the plates is doubled and the space between the plates is filled with a substance of dielectric constant 3, the capacity of the capacitor becomes
A)
\[\frac{1}{4}C\]
done
clear
B)
\[\frac{9}{2}C\]
done
clear
C)
\[\frac{2}{3}C\]
done
clear
D)
\[\frac{3}{2}C\]
done
clear
View Answer play_arrow
question_answer 26) The\[SI\]unit of electrostatic flux is
A)
\[V/{{m}^{2}}\]
done
clear
B)
V-m
done
clear
C)
V/m
done
clear
D)
\[V-{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 27) A wire of length 1 m and cross-sectional area \[4\times {{10}^{-9}}{{m}^{2}}\] is made of a metal of resistivity \[6\times {{10}^{-8}}\Omega -m.\]The potential difference needed to pass a current of 2 A in the wire is
A)
75 V
done
clear
B)
50 V
done
clear
C)
30 V
done
clear
D)
10 V
done
clear
View Answer play_arrow
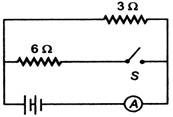
question_answer 28)
In the circuit shown, the resistance of the ammeter A and the battery are zero. Ammeter reads 0.2 A when the switch S is opened. When the switch S is closed, the ammeter will read
A)
0.1 A
done
clear
B)
0.2 A
done
clear
C)
0.3 A
done
clear
D)
0.6 A
done
clear
View Answer play_arrow
question_answer 29) The cold junction of a thermocouple is at \[0{}^\circ C\]. The thermo-emf \[\varepsilon ,\] in volt, generated in this thermocouple varies with temperature \[t\,\,{}^\circ C\] of the hot junction as \[\varepsilon =6+4t-\frac{{{t}^{2}}}{32}\] The neutral temperature of the thermocouple is
A)
\[100{}^\circ C\]
done
clear
B)
\[76{}^\circ C\]
done
clear
C)
\[64{}^\circ C\]
done
clear
D)
\[50{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 30) A bar magnet is placed in a non-uniform magnetic field. The bar magnet, in general, will experience
A)
a linear force and no torque
done
clear
B)
a torque and no linear force
done
clear
C)
both a torque and a linear force
done
clear
D)
neither a torque nor a linear force
done
clear
View Answer play_arrow
question_answer 31) Curie temperature is the temperature above which
A)
paramagnetic material becomes diamagnetic
done
clear
B)
paramagnetic material becomes ferromagnetic
done
clear
C)
diamagnetic material becomes paramagnetic
done
clear
D)
ferromagnetic material becomes paramagnetic
done
clear
View Answer play_arrow
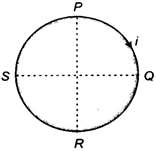
question_answer 32)
A circular coil PQRSP is placed in a uniform magnetic field. When a current flows through the coil, the force on the segment PQ is F. The force on the remaining segment QRSP is
A)
3F
done
clear
B)
-3F
done
clear
C)
F
done
clear
D)
-F
done
clear
View Answer play_arrow
question_answer 33) A charged particle moves in a circle of radius R under the influence of a constant transverse magnetic field. The time period of revolution is
A)
independent of R
done
clear
B)
proportional to R
done
clear
C)
proportional to\[\frac{1}{R}\]
done
clear
D)
proportional to \[\frac{1}{{{R}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 34) The eddy current loss in a transformer can be reduced by using a core material of
A)
high density
done
clear
B)
low density
done
clear
C)
high resistivity
done
clear
D)
low resistivity
done
clear
View Answer play_arrow
question_answer 35) The unit of mutual inductance of a coil can be expressed as
A)
Wb-A
done
clear
B)
Wb/A
done
clear
C)
Wb/m
done
clear
D)
Wb-m
done
clear
View Answer play_arrow
question_answer 36) If the values of resistance, inductive reactance and capacitive reactance in a series LCR AC circuit are \[3\,\Omega ,\] \[10\,\Omega \] and \[6\,\Omega \] respectively, the total impedance of the circuit is
A)
\[25\,\Omega \]
done
clear
B)
\[\sqrt{7}\,\Omega \]
done
clear
C)
\[5\,\Omega \]
done
clear
D)
\[19\,\Omega \]
done
clear
View Answer play_arrow
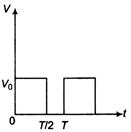
question_answer 37)
The potential difference V varies with time t as shown in the figure. The rms value of V is
A)
\[{{V}_{0}}\]
done
clear
B)
\[2{{V}_{0}}\]
done
clear
C)
\[{{V}_{0}}/2\]
done
clear
D)
\[{{V}_{0}}/\sqrt{2}\]
done
clear
View Answer play_arrow
question_answer 38) If the ionization potential of hydrogen atom is 13.6 eV, its energy in the n=3 is approximately
A)
-1.14 eV
done
clear
B)
-1.51 eV
done
clear
C)
-3.4 eV
done
clear
D)
-4.53 eV
done
clear
View Answer play_arrow
question_answer 39) The photoelectric work function of a metal is 3.3 eV. The threshold frequency for this metal is approximately
A)
\[3.3\times {{10}^{13}}\,Hz\]
done
clear
B)
\[8.0\times {{10}^{14}}\,Hz\]
done
clear
C)
\[1.65\times {{10}^{15}}\,Hz\]
done
clear
D)
\[9.9\times {{10}^{15}}\,Hz\]
done
clear
View Answer play_arrow
question_answer 40) A particle of mass\[10\times {{10}^{-12}}\] kg is moving with a velocity \[6\times {{10}^{-7}}\]m/s Its de-Broglie wavelength is nearly
A)
\[\text{1}{{\text{0}}^{\text{-20}}}\,\text{m}\]
done
clear
B)
\[\text{1}{{\text{0}}^{\text{-16}}}\,\text{m}\]
done
clear
C)
\[\text{1}{{\text{0}}^{\text{-12}}}\,\text{m}\]
done
clear
D)
\[\text{1}{{\text{0}}^{\text{-8}}}\,\text{m}\]
done
clear
View Answer play_arrow
question_answer 41) The mass numbers of nuclei A and B are respectively 135 and 5. The ratio of their radii is
A)
1 : 3
done
clear
B)
3 : 1
done
clear
C)
\[\sqrt{27}:1\]
done
clear
D)
\[1:\sqrt{27}\]
done
clear
View Answer play_arrow
question_answer 42) The resistances of a semiconductor and of a conductor
A)
increase with temperature
done
clear
B)
decrease with temperature
done
clear
C)
increases and decreases respectively with temperature
done
clear
D)
decreases and increases respectively with temperature
done
clear
View Answer play_arrow
question_answer 43)
The truth table A B Y 1 1 0 1 0 1 0 1 1 0 0 1
is of the
A)
NAND gate
done
clear
B)
OR gate
done
clear
C)
NOT gate
done
clear
D)
AND gate
done
clear
View Answer play_arrow
question_answer 44) The E and B vectors associated with an electromagnetic wave are
A)
parallel to each other and are in the same phase
done
clear
B)
parallel to each other and are in opposite phases
done
clear
C)
perpendicular to each other and are in opposite phases
done
clear
D)
perpendicular to each other and are in the same phase
done
clear
View Answer play_arrow
question_answer 45) The dimensions of energy per unit volume is the same as that of
A)
torque
done
clear
B)
pressure
done
clear
C)
acceleration
done
clear
D)
force
done
clear
View Answer play_arrow
question_answer 46) A uniform thin ring of mass 0.4 kg rolls without slipping on a horizontal surface with a linear velocity of 10 cm/s. The kinetic energy of the ring is
A)
\[4\times {{10}^{-3}}\]
done
clear
B)
\[4\times {{10}^{-2}}J\]
done
clear
C)
\[2\times {{10}^{-3}}J\]
done
clear
D)
\[2\times {{10}^{-2}}J\]
done
clear
View Answer play_arrow
question_answer 47) A car of mass 1000 kg travelling at a speed of 72 km/h hits a tree and comes to rest in 0.2 s. The average force on the car during the impact is
A)
\[36\times {{10}^{4}}N\]
done
clear
B)
\[1\times {{10}^{5}}N\]
done
clear
C)
\[36\times {{10}^{7}}N\]
done
clear
D)
\[36\times {{10}^{5}}N\]
done
clear
View Answer play_arrow
question_answer 48) The distance \[x\] in metre, travelled by a panicle in time t second is given by \[x=0.5{{t}^{3}}+2{{t}^{2}}\]. The acceleration of the particle at t = 2 s is
A)
\[2\,m/{{s}^{2}}\]
done
clear
B)
\[4\,m/{{s}^{2}}\]
done
clear
C)
\[8\,m/{{s}^{2}}\]
done
clear
D)
\[10\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 49) Assume the earth to be a sphere of radius R. If g is the acceleration due to gravity at any point on the earths surface, the mass of the earth is
A)
\[\frac{gR}{G}\]
done
clear
B)
\[\frac{{{g}^{2}}{{R}^{2}}}{G}\]
done
clear
C)
\[\frac{g{{R}^{2}}}{G}\]
done
clear
D)
\[\frac{{{g}^{2}}R}{G}\]
done
clear
View Answer play_arrow
question_answer 50) A body is projected with kinetic energy T at the angle of maximum range. Its kinetic energy at the highest point of the trajectory will be
A)
T
done
clear
B)
\[\text{T/}\sqrt{\text{2}}\]
done
clear
C)
T/2
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 51) Aqueous solutions of lithium salts are not good conductors of electricity, because of
A)
high hydration energy of \[L{{i}^{+}}\] ion
done
clear
B)
high ionization energy of \[L{{i}^{+}}\] ion
done
clear
C)
small size of \[L{{i}^{+}}\] ion
done
clear
D)
non-metallic character of \[L{{i}^{+}}\] ion
done
clear
View Answer play_arrow
question_answer 52) For the equation\[2A+BB{{A}_{2}}\], the equilibrium concentration of \[A,\,\,B,\,\,B{{A}_{2}}\] is 4, 2 and 2 respectively. The value of \[{{K}_{c}}\] will be
A)
\[0.0625\]
done
clear
B)
\[0.625\]
done
clear
C)
\[6.280\]
done
clear
D)
\[6.250\]
done
clear
View Answer play_arrow
question_answer 53) Relationship between equilibrium constant \[({{K}_{c}})\] and Gibbs energy \[(\Delta {{G}^{o}})\] at temperature \[T\] may be given by the expression
A)
\[\Delta {{G}^{o}}=-{{K}_{c}}\log RT\]
done
clear
B)
\[\Delta {{G}^{o}}=-RT\log {{K}_{c}}\]
done
clear
C)
\[\Delta {{G}^{o}}=-\frac{\log RT}{{{K}_{c}}}\]
done
clear
D)
\[\Delta {{G}^{o}}=-\log \frac{{{K}_{c}}}{RT}\]
done
clear
View Answer play_arrow
question_answer 54) Given for \[HA\] acid, \[{{K}_{a}}={{10}^{-6}}\] and for\[MOH\] base,\[{{K}_{b}}={{10}^{-6}}.\] The \[pH\] of \[0.1\,\,M\,\,MA\] salt solution will be
A)
5
done
clear
B)
7
done
clear
C)
9
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 55) The \[pH\] of an aqueous solution of a \[1\times {{10}^{-7}}M\] solution of \[HCl\] will be
A)
7
done
clear
B)
slightly less than 7
done
clear
C)
slightly greater than 7
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 56) What will be the concentration \[{{H}^{+}}\] ions in \[0.1\,\,M\] acetic acid and \[0.1\,\,M\] sodium acetate solution, if the dissociation constant of acetic acid is\[1.8\times {{10}^{-5}}\]?
A)
\[1.8\times {{10}^{-7}}\]
done
clear
B)
\[1.8\times {{10}^{-5}}\]
done
clear
C)
\[1.8\times {{10}^{-2}}\]
done
clear
D)
\[1.8\times {{10}^{-3}}\]
done
clear
View Answer play_arrow
question_answer 57) A system absorbs reversibly \[600\,\,J\] of heat and preforms \[250\,\,J\] of work. The increase in the internal energy of system is
A)
\[850\,\,J\]
done
clear
B)
\[250\,\,J\]
done
clear
C)
\[600\,\,J\]
done
clear
D)
\[350\,\,J\]
done
clear
View Answer play_arrow
question_answer 58) For the reaction \[A+2B\xrightarrow[{}]{{}}\]Products, the differential rate equation is
A)
\[-\frac{1}{2}\frac{d[A]}{dt}=\frac{-d[B]}{dt}=k[A]{{[B]}^{2}}\]
done
clear
B)
\[\frac{1}{2}-\frac{d[A]}{dt}=\frac{d[B]}{dt}=k[A]{{[B]}^{2}}\]
done
clear
C)
\[\frac{-d[A]}{dt}=-\frac{1}{2}\frac{d[B]}{dt}=k[A]{{[B]}^{2}}\]
done
clear
D)
\[\frac{d[A]}{dt}=\frac{1}{2}\frac{d[B]}{dt}=k[A]{{[B]}^{2}}\]
done
clear
View Answer play_arrow
question_answer 59) For reversible melting of ice at \[{{0}^{o}}C\] and \[1\,\,atm\]. pressure, the value of \[\Delta G\] will be
A)
\[\text{0(zero)}\]
done
clear
B)
\[\text{0(zero)}\]
done
clear
C)
\[\text{0(zero)}\]
done
clear
D)
\[\infty (\text{infinity})\]
done
clear
View Answer play_arrow
question_answer 60) The \[{{t}_{1/2}}\] of a reaction is halved as the initial concentration of the reactant is doubled. What is the order of reaction?
A)
First order
done
clear
B)
Zero order
done
clear
C)
Second order
done
clear
D)
Third order
done
clear
View Answer play_arrow
question_answer 61) Fog is a colloidal system of
A)
gas in liquid
done
clear
B)
liquid in gas
done
clear
C)
gas in gas
done
clear
D)
gas in solid
done
clear
View Answer play_arrow
question_answer 62) Semi-permeable membrane allows
A)
a solution to pass through it
done
clear
B)
solute to pass through it
done
clear
C)
solvent to pass through it
done
clear
D)
both the solute and solvent to pass through it
done
clear
View Answer play_arrow
question_answer 63) The molarity of a solution containing \[5\,\,g\] of \[NaOH\] in \[450\,\,mL\] solution will be
A)
\[0.189\,\,mol\,\,d{{m}^{-3}}\]
done
clear
B)
\[0.278\,\,mol\,\,d{{m}^{-3}}\]
done
clear
C)
\[0.556\,\,mol\,\,d{{m}^{-3}}\]
done
clear
D)
\[0.027\,\,mol\,\,d{{m}^{-3}}\]
done
clear
View Answer play_arrow
question_answer 64) The oxidation state of sulphur in \[{{H}_{2}}S{{O}_{5}}\] and of chromium in \[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\] respectively is
A)
\[8,\,\,6\]
done
clear
B)
\[4,\,\,6\]
done
clear
C)
\[8,\,\,8\]
done
clear
D)
\[6,\,\,6\]
done
clear
View Answer play_arrow
question_answer 65) If the cell reaction is spontaneous, then
A)
\[{{E}^{o}}=-ve\]
done
clear
B)
\[{{E}^{o}}=+ve\]
done
clear
C)
\[emf=+ve\]
done
clear
D)
\[(\Delta G+{{E}^{o}})=+ve\]
done
clear
View Answer play_arrow
question_answer 66) For the \[Zn-Cu\] cell,\[{{E}^{o}}=1.10\,\,V.\] If the reduction potential of \[C{{u}^{2+}}(aq)|Cu(s)\] couple is \[0.34\,\,V\], then the reduction potential of \[Z{{n}^{2+}}(aq)|Zn(s)\] couple will be
A)
\[-0.76\,\,V\]
done
clear
B)
\[0.76\,\,V\]
done
clear
C)
\[7.6\,\,V\]
done
clear
D)
\[0.38\,\,V\]
done
clear
View Answer play_arrow
question_answer 67) An example of a cyclic silicate is
A)
beryl
done
clear
B)
zeolite
done
clear
C)
talc
done
clear
D)
feldspar
done
clear
View Answer play_arrow
question_answer 68) Which halide is unknown?
A)
\[Tl(III)\]iodide
done
clear
B)
\[\ln (III)\]bromide
done
clear
C)
\[Ga(III)\]fluoride
done
clear
D)
\[B(III)\]chloride
done
clear
View Answer play_arrow
question_answer 69) Colourless ion-pair is
A)
\[C{{u}^{+}},\,\,Z{{n}^{2+}}\]
done
clear
B)
\[C{{u}^{2+}},\,\,Z{{n}^{2+}}\]
done
clear
C)
\[{{V}^{3+}},\,\,C{{r}^{3+}}\]
done
clear
D)
\[M{{n}^{3+}},\,\,F{{e}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 70) Similar sizes of second and third transition elements can be explained on the basis of
A)
inert-pair effect
done
clear
B)
screening effect
done
clear
C)
lanthanide contraction
done
clear
D)
increasing effective nuclear charge
done
clear
View Answer play_arrow
question_answer 71) A transitional metal complex adopts \[{{t}_{2g}}^{4}e_{g}^{2}\] configuration. The nature of ligand surrounding the ion is
A)
strong field
done
clear
B)
weak field
done
clear
C)
neutral
done
clear
D)
\[+ve\] field
done
clear
View Answer play_arrow
question_answer 72) Ether and benzene can be separated by
A)
filtration
done
clear
B)
distillation
done
clear
C)
crystallization
done
clear
D)
sublimation
done
clear
View Answer play_arrow
question_answer 73)
Consider the following compounds
A)
\[I>II>III>IV\]
done
clear
B)
\[IV>III>II>I\]
done
clear
C)
\[III>II>I>IV\]
done
clear
D)
\[III>IV>I>II\]
done
clear
View Answer play_arrow
question_answer 74) Ozonolysis of a hydrocarbon gives one mole of acetone and one mole of formaldehyde. The hydrocarbon is
A)
propene
done
clear
B)
2-methylpropene
done
clear
C)
2-methyl-2-butene
done
clear
D)
2-methyl-1-butene
done
clear
View Answer play_arrow
question_answer 75) In the free-radical halogenation of alkanes, chain propogating step is
A)
\[C{{l}_{2}}\xrightarrow{hv}2C{{l}^{\bullet }}\]
done
clear
B)
\[C{{H}_{4}}+C{{l}^{\bullet }}\xrightarrow{{}}C{{H}_{3}}Cl+{{H}^{\bullet }}\]
done
clear
C)
\[C{{H}_{4}}+C{{l}^{\bullet }}\xrightarrow{{}}CH_{3}^{\bullet }+HCl\]
done
clear
D)
\[CH_{3}^{\bullet }+C{{l}^{\bullet }}\xrightarrow{{}}C{{H}_{3}}Cl\]
done
clear
View Answer play_arrow
question_answer 76) Correct \[IUPAC\] name is
A)
3-methyl-2-ethylpentane
done
clear
B)
2-ethyl-3-methylpentane
done
clear
C)
3-ethyl-2-methylpentane
done
clear
D)
2-ethyl-2-methylpentane
done
clear
View Answer play_arrow
question_answer 77) By heating which mixture propane nitile be obtained?
A)
Ethyl alcohol\[+KCN\]
done
clear
B)
Propyl alcohol\[+KCN\]
done
clear
C)
Ethyl chloride\[+KCN\]
done
clear
D)
Propyl chloride\[+KCN\]
done
clear
View Answer play_arrow
question_answer 78)
A)
done
clear
B)
done
clear
C)
\[1:1\] mixture of both (a) and (b)
done
clear
D)
\[C{{H}_{3}}-CH=CH-C{{H}_{3}}\]
done
clear
View Answer play_arrow
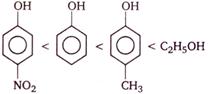
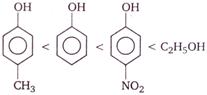
question_answer 79) Correct increasing order of acidity of the following phenols is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 80) \[p,\,\,s\] and \[t-\]alcohols can be distinguished by
A)
Reimer-Tiemann reaction
done
clear
B)
Tollens reagent
done
clear
C)
Lucas test
done
clear
D)
Lassaignes test
done
clear
View Answer play_arrow
question_answer 81) Major product of the reaction \[{{(C{{H}_{3}})}_{3}}C-Cl+{{C}_{2}}{{H}_{5}}ONa\xrightarrow{{}}\] would be
A)
\[{{(C{{H}_{3}})}_{2}}C-O{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[{{(C{{H}_{3}})}_{3}}C-{{C}_{2}}{{H}_{5}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}C=C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}-CH=CH-{{C}_{2}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 82) Which of the following compounds would exhibit highest dipole moment?
A)
Ethers
done
clear
B)
Alcohols
done
clear
C)
Carbonyl compounds
done
clear
D)
Carboxylic acids
done
clear
View Answer play_arrow
question_answer 83) Ketones can be prepared by
A)
Rosenmund reduction
done
clear
B)
Etard reaction
done
clear
C)
Cannizaro reaction
done
clear
D)
Friedel-Craft reaction
done
clear
View Answer play_arrow
question_answer 84) Biuret test is characteristic of compounds containing the functional group
A)
done
clear
B)
\[-N{{H}_{2}}\]
done
clear
C)
\[-CONH-\]
done
clear
D)
\[-C\equiv H\]
done
clear
View Answer play_arrow
question_answer 85) When propanoic acid is treated with aqueous sodium bicarbonate, \[C{{O}_{2}}\] is liberated. The carbon of \[C{{O}_{2}}\] comes from
A)
methyl group
done
clear
B)
carboxylic acid group
done
clear
C)
methylene group
done
clear
D)
bicarbonate
done
clear
View Answer play_arrow
question_answer 86)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 87) Benzene diazonium chloride on treatment with ethanol gives
A)
chlorobenzene
done
clear
B)
benzene
done
clear
C)
phenol
done
clear
D)
aniline
done
clear
View Answer play_arrow
question_answer 88) \[{{C}_{2}}{{H}_{5}}Cl\xrightarrow{N{{H}_{3}}}A\xrightarrow{{{C}_{2}}{{H}_{5}}Cl}B\xrightarrow{{{C}_{2}}{{H}_{5}}Cl}C\] \[A,\,\,B\] and \[C\] respectively are
A)
\[{{C}_{2}}{{H}_{5}}N{{H}_{2}},\,\,{{({{C}_{2}}{{H}_{5}})}_{2}}NH,\,\,{{({{C}_{2}}{{H}_{5}})}_{3}}N\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}N{{H}_{2}},\,\,{{C}_{2}}{{H}_{5}}NH-Cl,\,\,{{C}_{2}}{{H}_{5}}-NC{{l}_{2}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}N{{H}_{2}},\,\,C{{H}_{2}}=C{{H}_{2}},\]\[Cl-C{{H}_{2}}-C{{H}_{2}}-{{C}_{2}}{{H}_{5}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}N{{H}_{2}},\,\,{{({{C}_{2}}{{H}_{5}})}_{3}}N,\,\,{{({{C}_{2}}{{H}_{5}})}_{2}}NH\]
done
clear
View Answer play_arrow
question_answer 89) Heating a mixture of a primary alkyl amine and chloroform with ethanolic potassium hydroxide gives
A)
alkyl chloride
done
clear
B)
alkyl alcohol
done
clear
C)
alkyl isocyanide
done
clear
D)
alkene
done
clear
View Answer play_arrow
question_answer 90) Weakest intermolecular forces are present in
A)
neoprene
done
clear
B)
terylene
done
clear
C)
polystyrene
done
clear
D)
bakelite
done
clear
View Answer play_arrow
question_answer 91) Which among the following is a tranquilizer?
A)
Equanil
done
clear
B)
Promethazine
done
clear
C)
Omeprazole
done
clear
D)
Cimetidine
done
clear
View Answer play_arrow
question_answer 92) If the de-Broglie wavelength of the fourth Bohr orbit of hydrogen atom is\[4\overset{\text{o}}{\mathop{\text{A}}}\,\], the circumference of the orbit will be
A)
\[4\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[4\,\,nm\]
done
clear
C)
\[16\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[16\,\,nm\]
done
clear
View Answer play_arrow
question_answer 93) The photoelectric work function depends upon the
A)
nature of metal
done
clear
B)
intensity of radiation
done
clear
C)
time of radiation
done
clear
D)
kinetic energy of photoelectron
done
clear
View Answer play_arrow
question_answer 94) The value of \[n=2\] and \[l=1\], the type of orbital would be
A)
\[2s\]
done
clear
B)
\[2p\]
done
clear
C)
\[1s\]
done
clear
D)
\[3p\]
done
clear
View Answer play_arrow
question_answer 95) The modern Periodic Table has been divided into
A)
seven periods: three short and four long
done
clear
B)
nine periods: six short and three long
done
clear
C)
eighteen periods: ten short and eight long
done
clear
D)
nine periods from zero to VIII
done
clear
View Answer play_arrow
question_answer 96) Among \[K,\,\,{{K}^{+}},\,\,S{{r}^{2+}},\,\,Ar,\] which atom/ion will have the smallest radius?
A)
\[{{K}^{+}}\]
done
clear
B)
\[S{{r}^{2+}}\]
done
clear
C)
\[Ar\]
done
clear
D)
\[K\]
done
clear
View Answer play_arrow
question_answer 97) Which of the following is paramagnetic?
A)
\[O_{2}^{-}\]
done
clear
B)
\[C{{N}^{-}}\]
done
clear
C)
\[CO\]
done
clear
D)
\[N{{O}^{+}}\]
done
clear
View Answer play_arrow
question_answer 98) The fusible mass obtained from high melting oxide in the gangue is called
A)
flux
done
clear
B)
slag
done
clear
C)
matte
done
clear
D)
matrix
done
clear
View Answer play_arrow
question_answer 99) The melting point of \[NaCl\] is high because
A)
the distance between the ions is large
done
clear
B)
repulsion exists within \[NaCl\] lattice
done
clear
C)
lattice energy is high
done
clear
D)
size of sodium as well as chlorine is big
done
clear
View Answer play_arrow
question_answer 100) The bond angle in water molecule is less than in ammonia molecule, due to the
A)
presence of two lone pairs in \[{{H}_{2}}O\] molecule
done
clear
B)
presence of three lone pairs in \[{{H}_{2}}O\] molecule
done
clear
C)
presence of two lone pairs in \[N{{H}_{3}}\] molecule
done
clear
D)
high electronegativity of oxygen atom
done
clear
View Answer play_arrow
question_answer 101) The endometrium is the lining of
A)
bladder
done
clear
B)
vagina
done
clear
C)
uterus
done
clear
D)
oviduct
done
clear
View Answer play_arrow
question_answer 102) GIFT
A)
transfer of zygote into the fallopian tube
done
clear
B)
transfer of embryo into the uterus
done
clear
C)
transfer of mixture of sperms and ova into the fallopian tube
done
clear
D)
transfer of mixture of sperms and ova into the uterus
done
clear
View Answer play_arrow
question_answer 103) Fusion of dissimilar gametes is known as
A)
fertilization
done
clear
B)
dichogamy
done
clear
C)
autogamy
done
clear
D)
allogamy
done
clear
View Answer play_arrow
question_answer 104) The process of translation is
A)
DNA synthesis
done
clear
B)
RNA synthesis
done
clear
C)
protein synthesis
done
clear
D)
ribosome synthesis
done
clear
View Answer play_arrow
question_answer 105) The codon for anti-codon\[\text{3 }\!\!\!\!\text{ UUUA5 }\!\!\!\!\text{ }\] is
A)
\[\text{3 }\!\!\!\!\text{ -UUUA-5 }\!\!\!\!\text{ }\]
done
clear
B)
\[\text{5 }\!\!\!\!\text{ -UAAA-3 }\!\!\!\!\text{ }\]
done
clear
C)
\[\text{5 }\!\!\!\!\text{ -AAAU-3 }\!\!\!\!\text{ }\]
done
clear
D)
\[\text{3 }\!\!\!\!\text{ -UAAU-5 }\!\!\!\!\text{ }\]
done
clear
View Answer play_arrow
question_answer 106) A kind of biotechnology involving manipulation of DNA is
A)
DNA replication
done
clear
B)
genetic engineering
done
clear
C)
denaturation
done
clear
D)
renaturation
done
clear
View Answer play_arrow
question_answer 107) What is true for plasmid?
A)
Found in viruses
done
clear
B)
Contains genes for vital activities
done
clear
C)
Part of nuclear chromosome
done
clear
D)
Widely used in gene transfer
done
clear
View Answer play_arrow
question_answer 108) DNA fingerprinting technique was discovered by
A)
Wilmut
done
clear
B)
A. Jeffreys
done
clear
C)
Ethoven
done
clear
D)
Kary Mullis
done
clear
View Answer play_arrow
question_answer 109) The term Vaccine was introduced by
A)
Jenner
done
clear
B)
Koch
done
clear
C)
Pasteur
done
clear
D)
Jointly by Koch and Pasteur
done
clear
View Answer play_arrow
question_answer 110) The immune system is made of
A)
humoral system
done
clear
B)
humoral and cell mediated systems
done
clear
C)
humoral and fibrous systems
done
clear
D)
antigen induced antibodies
done
clear
View Answer play_arrow
question_answer 111) Minamata disease is caused due to presence of...... in water.
A)
cadmium
done
clear
B)
lead
done
clear
C)
arsenic
done
clear
D)
mercury
done
clear
View Answer play_arrow
question_answer 112) Amniocentesis detects
A)
deformity in brain
done
clear
B)
deformity in heart
done
clear
C)
hereditary disease
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 113) Metastasis is associated with
A)
malignant tumours
done
clear
B)
benign tumours
done
clear
C)
Both a and b
done
clear
D)
crown gall tumour
done
clear
View Answer play_arrow
question_answer 114) Which was absent in the atmosphere at the time of origin of life?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[{{H}_{2}}\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
\[C{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 115) Organic compounds first evolved in earth required for origin of life were
A)
urea and amino acids
done
clear
B)
proteins and nucleic acids
done
clear
C)
proteins and amino acids
done
clear
D)
urea and nucleic acids
done
clear
View Answer play_arrow
question_answer 116) Homologous organs indicate the
A)
convergent evolution
done
clear
B)
parallel evolution
done
clear
C)
common descendent
done
clear
D)
natural selection
done
clear
View Answer play_arrow
question_answer 117) The first organism to be found on a bare rock is
A)
moss
done
clear
B)
alga
done
clear
C)
lichen
done
clear
D)
fem
done
clear
View Answer play_arrow
question_answer 118) The phrase Omnis cellula e cellula was given by
A)
Virchow
done
clear
B)
Pasteur
done
clear
C)
Schleilden
done
clear
D)
Brown
done
clear
View Answer play_arrow
question_answer 119) Starfish belongs to the class
A)
Pisces
done
clear
B)
Cephalopoda
done
clear
C)
Asteroidea
done
clear
D)
Ophuroidea
done
clear
View Answer play_arrow
question_answer 120) Which of the following is not an insect?
A)
Ant
done
clear
B)
Mosquito
done
clear
C)
Spider
done
clear
D)
Locusts
done
clear
View Answer play_arrow
question_answer 121) The canal system is characteristic feature of
A)
sponges
done
clear
B)
helminthes
done
clear
C)
echinoderms
done
clear
D)
coelenterates
done
clear
View Answer play_arrow
question_answer 122) In Protozoa, like Amoeba and Paramecium, the organ found for osmoregulation is
A)
nucleus
done
clear
B)
food vacuole
done
clear
C)
mitochondria
done
clear
D)
contractile vacuole
done
clear
View Answer play_arrow
question_answer 123) Which of the following organisms is pseudocoelomate?
A)
Hookworm
done
clear
B)
Liver fluke
done
clear
C)
Jellyfish
done
clear
D)
Leech
done
clear
View Answer play_arrow
question_answer 124) Mammals heart is
A)
myogenic
done
clear
B)
neurogenic
done
clear
C)
voluntary
done
clear
D)
sympathetic
done
clear
View Answer play_arrow
question_answer 125) Natural parthenogenesis is found in
A)
housefly
done
clear
B)
honey bee
done
clear
C)
Drosophila
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 126) Which of the following snake is non-poisonous?
A)
Cobra
done
clear
B)
Krait
done
clear
C)
Viper
done
clear
D)
Pyrhon
done
clear
View Answer play_arrow
question_answer 127) A group of animals having marsupium is
A)
Monotremata
done
clear
B)
Eutheria
done
clear
C)
Metatheria
done
clear
D)
Pantotheria
done
clear
View Answer play_arrow
question_answer 128) Which of the following belongs to Phylum- Arthropoda?
A)
Starfish
done
clear
B)
Goldfish
done
clear
C)
Silver fish
done
clear
D)
Cuttlefish
done
clear
View Answer play_arrow
question_answer 129) Browsing by animals is an example of
A)
Parasitism
done
clear
B)
predation
done
clear
C)
Commensalism
done
clear
D)
ferns
done
clear
View Answer play_arrow
question_answer 130) Intermediate community between pioneer and climax communities is called
A)
seral community
done
clear
B)
biotic community
done
clear
C)
temporary community
done
clear
D)
ecosere
done
clear
View Answer play_arrow
question_answer 131) In parasitic food chain, the pyramid of number is
A)
inverted
done
clear
B)
upright
done
clear
C)
linear
done
clear
D)
Both a and b
done
clear
View Answer play_arrow
question_answer 132) Ten per cent law of energy transfer in a food chain is given by
A)
Schimper
done
clear
B)
Eiton
done
clear
C)
Haeckel
done
clear
D)
Lindemann
done
clear
View Answer play_arrow
question_answer 133) In a food chain the largest population is that of
A)
producers
done
clear
B)
decomposers
done
clear
C)
secondary consumers
done
clear
D)
primary consumers
done
clear
View Answer play_arrow
question_answer 134) Initiation codon is
A)
AUG
done
clear
B)
AGU
done
clear
C)
AAU
done
clear
D)
AUA
done
clear
View Answer play_arrow
question_answer 135) Apoenzyme is
A)
protein
done
clear
B)
carbohydrate
done
clear
C)
vitamin
done
clear
D)
amino acid
done
clear
View Answer play_arrow
question_answer 136) Which amino acids are present in histones?
A)
Lysine and histidine
done
clear
B)
Valine and histidine
done
clear
C)
Arginine and lysine
done
clear
D)
Arginine and histidine
done
clear
View Answer play_arrow
question_answer 137) Glycogenolysis involves
A)
conversion of sugar into glycogen
done
clear
B)
oxidation of sugar
done
clear
C)
conversion of glycogen into sugar
done
clear
D)
conversion of glycogen into fat
done
clear
View Answer play_arrow
question_answer 138) The two strands of the DNA double helix are
A)
held together by
done
clear
B)
hydrogen bonds
done
clear
C)
hydrophobic bonds
done
clear
D)
peptide bonds phosphodiester bonds
done
clear
View Answer play_arrow
question_answer 139) A polygenic inheritance in human beings is
A)
skin colour
done
clear
B)
sickle cell anaemia
done
clear
C)
colour blindness
done
clear
D)
phenylketonuria
done
clear
View Answer play_arrow
question_answer 140) A woman with albinic father marries an albinic man. The proportion of her progeny is
A)
2 normal: 1 albinic
done
clear
B)
all normal
done
clear
C)
all albinic
done
clear
D)
1 normal: 1 albinic
done
clear
View Answer play_arrow
question_answer 141) A cross between pure tall pea plant with green pods and dwarf pea with yellow pods will produce tall F2 plants, out of 16
A)
15
done
clear
B)
13
done
clear
C)
12
done
clear
D)
7
done
clear
View Answer play_arrow
question_answer 142) Dihybrid cross is related to the principle of
A)
dominance
done
clear
B)
segregation
done
clear
C)
independent assortment
done
clear
D)
Hybridization
done
clear
View Answer play_arrow
question_answer 143) Emasculation is related to
A)
pure line
done
clear
B)
mass selection
done
clear
C)
clonal selection
done
clear
D)
hybridization
done
clear
View Answer play_arrow
question_answer 144) Which sound producing organ is found in bird?
A)
Pharynx
done
clear
B)
Larynx
done
clear
C)
Syrinx
done
clear
D)
Trachea
done
clear
View Answer play_arrow
question_answer 145) During expiration, the diaphragm becomes
A)
normal
done
clear
B)
flattened
done
clear
C)
dome-shaped
done
clear
D)
oblique
done
clear
View Answer play_arrow
question_answer 146) Serum is
A)
blood without fibrinogen
done
clear
B)
lymph without corpuscles
done
clear
C)
blood without corpuscles and fibrinogen
done
clear
D)
lymph
done
clear
View Answer play_arrow
question_answer 147) In a healthy adult man the normal diastolic pressure is
A)
90 mm Hg
done
clear
B)
120 mm Hg
done
clear
C)
80 mm Hg
done
clear
D)
100 mm Hg
done
clear
View Answer play_arrow
question_answer 148) The joint of radio-ulna with the upper arm is
A)
hinge joint
done
clear
B)
pivot joint
done
clear
C)
socket joint
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 149) Sertoli cells are found in
A)
heart
done
clear
B)
liver
done
clear
C)
germinal epithelium
done
clear
D)
seminiferous tubules
done
clear
View Answer play_arrow
question_answer 150) At the time of implantation, the human embryo is called
A)
embryo
done
clear
B)
blastocyst
done
clear
C)
zygote
done
clear
D)
foetus
done
clear
View Answer play_arrow
question_answer 151) Nucleic acid segment tagged with a radiactive molecule is called
A)
clone
done
clear
B)
probe
done
clear
C)
plasmid
done
clear
D)
vector
done
clear
View Answer play_arrow
question_answer 152) GM brinjal in India has been developed for resistance against
A)
virus
done
clear
B)
bacteria
done
clear
C)
fungi
done
clear
D)
insects
done
clear
View Answer play_arrow
question_answer 153) The drug cyclosporin used for organ transplant patients is obtained from a
A)
bacterium
done
clear
B)
fungus
done
clear
C)
virus
done
clear
D)
plant
done
clear
View Answer play_arrow
question_answer 154) A common biocontrol agent for the control of plant diseases
A)
Agrobacterium
done
clear
B)
Glomus
done
clear
C)
Trichoderma
done
clear
D)
Baculovirus
done
clear
View Answer play_arrow
question_answer 155) Powdery mildew of wheat is caused by a species of
A)
Puccinia
done
clear
B)
Erysiphe
done
clear
C)
Ustilago
done
clear
D)
Albugo
done
clear
View Answer play_arrow
question_answer 156) In paddy fields biological nitrogen fixation is chiefly brought by
A)
cyanobacteria
done
clear
B)
green algae
done
clear
C)
mycorrhiza
done
clear
D)
Rhizobium
done
clear
View Answer play_arrow
question_answer 157) An important drug is obtained from the bark of
A)
Papaver
done
clear
B)
Cinchona
done
clear
C)
Withania
done
clear
D)
Momordica
done
clear
View Answer play_arrow
question_answer 158) Germplasm conservation in situ is done by
A)
biosphere reserves
done
clear
B)
botanical gardens
done
clear
C)
germplasm banks
done
clear
D)
pollen banks
done
clear
View Answer play_arrow
question_answer 159) In five-kingdom classification, Euglena is placed in
A)
Monera
done
clear
B)
Protista
done
clear
C)
Fungi
done
clear
D)
Animalia
done
clear
View Answer play_arrow
question_answer 160) Nucleic acid is absent in
A)
virus
done
clear
B)
viroid
done
clear
C)
prion
done
clear
D)
mycoplasma
done
clear
View Answer play_arrow
question_answer 161) In most fungi, cell wall is chiefly made of
A)
cellulose
done
clear
B)
chitin
done
clear
C)
protein
done
clear
D)
lipid
done
clear
View Answer play_arrow
question_answer 162) Sporophyte is not an independent generation in
A)
bryophytes
done
clear
B)
pteridophytes
done
clear
C)
gymnospenns
done
clear
D)
angiosperms
done
clear
View Answer play_arrow
question_answer 163) Both pteridophytes and gymnospenns have
A)
seeds
done
clear
B)
independent gametophytes
done
clear
C)
archegonia
done
clear
D)
ovules
done
clear
View Answer play_arrow
question_answer 164) Heterocysts are present in
A)
Riccia
done
clear
B)
Ulothrix
done
clear
C)
Albugo
done
clear
D)
Nostoc
done
clear
View Answer play_arrow
question_answer 165) In Albugo, sexual reproduction results in the formation of
A)
zygospore
done
clear
B)
oospore
done
clear
C)
basidiospore
done
clear
D)
teliospore
done
clear
View Answer play_arrow
question_answer 166) Double fertilization occurs in
A)
Riccia
done
clear
B)
Pteridium
done
clear
C)
Cycas
done
clear
D)
Capsella
done
clear
View Answer play_arrow
question_answer 167) Velamen is present in roots of
A)
Vanda
done
clear
B)
Rhizophora
done
clear
C)
Asparagus
done
clear
D)
Maize
done
clear
View Answer play_arrow
question_answer 168) In Ruscus, the stem is a
A)
phyllode
done
clear
B)
cladode
done
clear
C)
offset
done
clear
D)
sucker
done
clear
View Answer play_arrow
question_answer 169) In turmeric, stem is a
A)
tuber
done
clear
B)
bulb
done
clear
C)
rhizome
done
clear
D)
corm
done
clear
View Answer play_arrow
question_answer 170) Catkin inflorescence is found in
A)
wheat
done
clear
B)
oat
done
clear
C)
mulberry
done
clear
D)
fig
done
clear
View Answer play_arrow
question_answer 171) A racemose type of inflorescence with its main axis almost flat is called
A)
corymb
done
clear
B)
umbel
done
clear
C)
spike
done
clear
D)
capitulum
done
clear
View Answer play_arrow
question_answer 172) Epigynous flowers are present in
A)
mustard
done
clear
B)
brinjal
done
clear
C)
China rose
done
clear
D)
cucumber
done
clear
View Answer play_arrow
question_answer 173) A small, dry, one-seeded fruit with its pericarp fused with the seed-coat, developing from a monocarpellary gynoecium is called
A)
cypsela
done
clear
B)
siliqua
done
clear
C)
caryopsis
done
clear
D)
samara
done
clear
View Answer play_arrow
question_answer 174) Spathe is present in the flowers of
A)
banana
done
clear
B)
rice
done
clear
C)
marigold
done
clear
D)
sunflower
done
clear
View Answer play_arrow
question_answer 175) In Dianthus, placentation is
A)
basal
done
clear
B)
free central
done
clear
C)
axile
done
clear
D)
marginal
done
clear
View Answer play_arrow
question_answer 176) Ovary is half-inferior in the flower of
A)
apple
done
clear
B)
guava
done
clear
C)
peach
done
clear
D)
garlic
done
clear
View Answer play_arrow
question_answer 177) The term keel is used for special type of
A)
sepals
done
clear
B)
petals
done
clear
C)
stamens
done
clear
D)
carpels
done
clear
View Answer play_arrow
question_answer 178) Polyadelphous stamens are found in
A)
cotton
done
clear
B)
sunflower
done
clear
C)
grain
done
clear
D)
lemon
done
clear
View Answer play_arrow
question_answer 179) Replum is the characteristic feature of the ovary of
A)
Asteraceae
done
clear
B)
Brassicaceae
done
clear
C)
Malvaceae
done
clear
D)
Liliaceae
done
clear
View Answer play_arrow
question_answer 180) Coffee and quinine are obtained from the
A)
Leguminosae
done
clear
B)
Asteraceae
done
clear
C)
Rubiaceae
done
clear
D)
Poaceae
done
clear
View Answer play_arrow
question_answer 181) Which of the following includes largest number of genera and species of plants?
A)
Brassicaceae
done
clear
B)
Liliaceae
done
clear
C)
Malvaceae
done
clear
D)
Asteraceae
done
clear
View Answer play_arrow
question_answer 182) Flowers are zygomorphic in
A)
mustard
done
clear
B)
radish
done
clear
C)
lily
done
clear
D)
candytuft
done
clear
View Answer play_arrow
question_answer 183) An old trunk of shisham (Dalbergia sissoo) tree would possess maximum amount of
A)
primary xylem
done
clear
B)
secondary xylem
done
clear
C)
primary phloem
done
clear
D)
secondary cortex
done
clear
View Answer play_arrow
question_answer 184) Vascular bundles are arranged in a ring in stem of
A)
wheat
done
clear
B)
rice
done
clear
C)
gram
done
clear
D)
maize
done
clear
View Answer play_arrow
question_answer 185) Kranz anatomy can be observed in leaves of
A)
sorghum
done
clear
B)
spinach
done
clear
C)
mustard
done
clear
D)
tulip
done
clear
View Answer play_arrow
question_answer 186) In higher plants, transport of food material occurs through
A)
companion cells
done
clear
B)
sieve elements
done
clear
C)
tracheids
done
clear
D)
transfusion tissue
done
clear
View Answer play_arrow
question_answer 187) The term bark refers to
A)
phellem, phelloderm and vascular cambium
done
clear
B)
periderm and secondary xylem
done
clear
C)
cork cambium and cork
done
clear
D)
phellogen, phellem, phelloderm and secondary phloem
done
clear
View Answer play_arrow
question_answer 188) In angiosperms, female gametophyte is represented by
A)
ovule
done
clear
B)
nucellus
done
clear
C)
megaspore mother cell
done
clear
D)
embryo sac
done
clear
View Answer play_arrow
question_answer 189) The transfer of pollen grains from the anther to the stigma of another flower of the same plant is called
A)
geitonogamy
done
clear
B)
autogamy
done
clear
C)
karyogamy
done
clear
D)
xenogamy
done
clear
View Answer play_arrow
question_answer 190) Development of embryo from nucellar cell is common in many varieties of
A)
citrus
done
clear
B)
sunflower
done
clear
C)
oat
done
clear
D)
marigold
done
clear
View Answer play_arrow
question_answer 191) Endosperm is not completely consumed during embryo development in seeds of
A)
gram
done
clear
B)
pea
done
clear
C)
bean
done
clear
D)
castor
done
clear
View Answer play_arrow
question_answer 192) Developing pollen grains in microsporangium get their nourishment from
A)
epidermis
done
clear
B)
endothecium
done
clear
C)
middle layer
done
clear
D)
tapetum
done
clear
View Answer play_arrow
question_answer 193) Light reaction in stroma lamellae of the chloroplast results in the formation of
A)
\[NADP{{H}_{2}}\]
done
clear
B)
\[ATP+NADP{{H}_{2}}\]
done
clear
C)
ATP
done
clear
D)
\[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 194) One of the commonly used plant growth hormone in tea plantations is
A)
ABA
done
clear
B)
zeatin
done
clear
C)
\[{{I}_{AA}}\]
done
clear
D)
ethylene
done
clear
View Answer play_arrow
question_answer 195) Leghaemoglobin in root nodules of legumes
A)
protects nitrogenase
done
clear
B)
converts \[{{N}_{3}}\] to \[N{{H}_{3}}\]
done
clear
C)
oxidizes \[N{{O}_{2}}\] to \[N{{O}_{3}}\]
done
clear
D)
helps in development of infection threads
done
clear
View Answer play_arrow
question_answer 196) The chief sinks for the mineral elements are
A)
senescent leaves
done
clear
B)
ripe fruits
done
clear
C)
lateral meristems
done
clear
D)
bark
done
clear
View Answer play_arrow
question_answer 197) In leaves of \[{{C}_{4}}\] plants malic acid synthesis during \[C{{O}_{2}}\] fixation occurs in
A)
bundle sheath
done
clear
B)
mesophyll
done
clear
C)
epidermis
done
clear
D)
guard cells
done
clear
View Answer play_arrow
question_answer 198) Which of the following helps in maintenance of cell shape
A)
plasmalemma
done
clear
B)
endoplasmic reticulum
done
clear
C)
cytoskeleton
done
clear
D)
mesosome
done
clear
View Answer play_arrow
question_answer 199) Membrane is absent in
A)
nucleus
done
clear
B)
nucleolus
done
clear
C)
vacuole
done
clear
D)
lysosome
done
clear
View Answer play_arrow
question_answer 200) A suitable vector gene cloning in higher
A)
organisms is
done
clear
B)
baculovirus
done
clear
C)
retrovirus
done
clear
D)
Salmonella typhimurium Neurospora crassa
done
clear
View Answer play_arrow
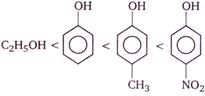
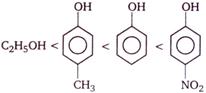
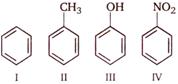 Correct order of their reactivity in electrophilic substitution reactions would be
Correct order of their reactivity in electrophilic substitution reactions would be
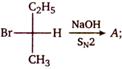 A is
A is