question_answer 1) If anybody is rolling on surface, then its total energy will be
A)
\[\frac{1}{2}m{{v}^{2}}\,+\,\frac{1}{2}I{{\omega }^{2}}\]
done
clear
B)
\[\frac{1}{2}m{{v}^{2}}\,-\,\frac{G{{m}_{1}}{{m}_{2}}}{R}\]
done
clear
C)
\[\frac{1}{2}m{{v}^{2}}\,-\,\frac{1}{2}\,m{{R}^{2}}{{\omega }^{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 2) A body of mass 5 kg is moving with velocity of 20 m/s. If the force of 200 N acts upon the body for 10 s, then finally the velocity of body will be
A)
150 m/s
done
clear
B)
220 m/s
done
clear
C)
200 m/s
done
clear
D)
240 m/s
done
clear
View Answer play_arrow
question_answer 3) What will be the moment of inertia of a ring about tangential axis in its plane?
A)
\[M{{R}^{2}}\]
done
clear
B)
\[2M{{R}^{2}}\]
done
clear
C)
\[\frac{3}{2}M{{R}^{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 4) The escape velocity of an object on a planet whose gravity value is 2 times as on earth and whose radius is 2 times that of earth will be
A)
\[2{{V}_{e}}\]
done
clear
B)
\[3{{V}_{e}}\]
done
clear
C)
\[4{{V}_{e}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 5) Dimensions of universal gravitational constant will be
A)
\[[M{{L}^{3}}{{T}^{-2}}]\]
done
clear
B)
\[[M{{L}^{-1}}{{L}^{3}}{{T}^{-2}}]\]
done
clear
C)
\[[{{M}^{-1}}{{L}^{2}}{{T}^{2}}]\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 6) If y = 2.4\[\eta \]the poisson ratio is
A)
\[-1\]
done
clear
B)
0.2
done
clear
C)
0.1
done
clear
D)
\[-0.25\]
done
clear
View Answer play_arrow
question_answer 7) Which of the following quantity are not same?
A)
Planck's constant and angular momentum
done
clear
B)
Work and energy
done
clear
C)
Pressure and Young's modulus
done
clear
D)
Torque of force and moment of inertia
done
clear
View Answer play_arrow
question_answer 8) The ratio of traveled distances by freely falling body in first, second and third seconds will be
A)
5 : 3 : 1
done
clear
B)
1 : 4 : 9
done
clear
C)
1 : 3 : 5
done
clear
D)
9 : 4 : 1
done
clear
View Answer play_arrow
question_answer 9) What will be the value of gravitational acceleration at height h from surface of earth? If h>>R, where R is radius of earth and gravitational acceleration at surface of earth is g.
A)
\[\frac{g}{{{\left( 1\,+\,\frac{h}{R} \right)}^{2}}}\]
done
clear
B)
\[g\left( 1-\frac{2h}{g} \right)\]
done
clear
C)
\[\frac{g}{{{\left( 1-\frac{h}{R} \right)}^{2}}}\]
done
clear
D)
\[g{{\left( 1-\frac{h}{R} \right)}^{{}}}\]
done
clear
View Answer play_arrow
question_answer 10) Two trains of length 50 m are crossing each other. If velocities in opposite directions are 10 m/s and 15 m/s, then time taken to cross each other will be
A)
2s
done
clear
B)
4s
done
clear
C)
6s
done
clear
D)
8s
done
clear
View Answer play_arrow
question_answer 11) Two springs of force constants k1 = 500 N/m and \[{{k}_{2}}=3000\text{ }N/m\]stretched by same force. Therefore ratio of their potential energies will be
A)
2 : 1
done
clear
B)
1 : 2
done
clear
C)
4 : 1
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 12) If potential and current are\[V=(100\pm 0.5)\]volt and\[I=(10\pm 0.2)\text{ }A\]respectively, then value of resistance will be
A)
(10 ± 0.7)\[\Omega \]
done
clear
B)
(5 ± 2)\[\Omega \]
done
clear
C)
(0.1 ± 2)\[\Omega \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 13) The range of projectile is four times of its maximum height, then angle of projection will be
A)
\[60{}^\circ \]
done
clear
B)
\[45{}^\circ \]
done
clear
C)
\[30{}^\circ \]
done
clear
D)
\[75{}^\circ \]
done
clear
View Answer play_arrow
question_answer 14) What will be the orbital velocity of planet revolves near earth (where R is radius of earth and g is gravitational acceleration)?
A)
\[\sqrt{Rg}\]
done
clear
B)
\[\sqrt{2Rg}\]
done
clear
C)
\[2\sqrt{Rg}\]
done
clear
D)
\[\sqrt{3Rg}\]
done
clear
View Answer play_arrow
question_answer 15) If bullets of mass m strikes on a wall at the rate of 27 per second, then what will be the force applied on the wall?
A)
mnv
done
clear
B)
4mnv
done
clear
C)
2mnv
done
clear
D)
\[\frac{mnv}{2}\]
done
clear
View Answer play_arrow
question_answer 16) If the length of simple pendulum increases 1%, then change in time period will be
A)
0.5%
done
clear
B)
1%
done
clear
C)
2%
done
clear
D)
0.2%
done
clear
View Answer play_arrow
question_answer 17) Two bodies of mass 1 kg are placed at a distance of 1 m have each other. The force between two bodies will be
A)
equal to G
done
clear
B)
equal to gravitation
done
clear
C)
equal to G / 2
done
clear
D)
None of these
done
clear
View Answer play_arrow
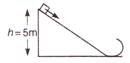
question_answer 18)
As per given figure to complete the circular loop what should be its radius, if initial height is 5m?
A)
4 m
done
clear
B)
3 m
done
clear
C)
2.5 m
done
clear
D)
2 m
done
clear
View Answer play_arrow
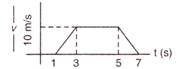
question_answer 19)
The particle moves according to given velocity-time graph. The ratio of travelling distance in last 2s and 7s will be
A)
\[\frac{1}{4}\]
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
\[\frac{1}{8}\]
done
clear
D)
\[\frac{1}{6}\]
done
clear
View Answer play_arrow
question_answer 20) On playing sitar the nature of wave on the reaching the observer will be
A)
transverse and progressive
done
clear
B)
longitudinal and progressive
done
clear
C)
transverse and stationary
done
clear
D)
longitudinal and stationary
done
clear
View Answer play_arrow
question_answer 21) The gravitation of earth is 10 m/s. If the mass of earth is 80 times, the mass of moon and radius of earth is 4 times the radius of moon, then the gravitation at moon will be
A)
\[\text{1 }m/{{s}^{2}}\]
done
clear
B)
\[2\text{ }m/{{s}^{2}}\]
done
clear
C)
\[\text{3 }m/{{s}^{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 22) The maximum acceleration in SHM will be
A)
at amplitude
done
clear
B)
at equilibrium position
done
clear
C)
at any point
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 23) The value of \[{{\varepsilon }_{0}}\] will be
A)
\[8.85\,\times \,{{10}^{-12}}{{C}^{2}}/N-{{m}^{2}}\]
done
clear
B)
\[8.85\,\times \,{{10}^{-12}}\,Fermi\]
done
clear
C)
\[9\,\times \,{{10}^{-13}}\,{{C}^{2}}/N-{{m}^{2}}\]
done
clear
D)
\[9\,\times \,{{10}^{-13}}\,Fermi\]
done
clear
View Answer play_arrow
question_answer 24) Rain water falls horizontally downwards with velocity v. When the velocity of air in horizontal direction is u, then water is store at the rate R m3/s. When the velocity of air becomes 2u, the rate of store of water will be
A)
R
done
clear
B)
R/2
done
clear
C)
2R
done
clear
D)
R\[\frac{\sqrt{4{{u}^{2}}\,+\,{{v}^{2}}}}{\sqrt{{{u}^{2}}+{{v}^{2}}}}\]
done
clear
View Answer play_arrow
question_answer 25) In Carnot cycle, the temperature of source is 500 K. If engine lakes in 300 cal of heat from a source and gives 150 cal of heat to a sink. The temperature of sink will be
A)
500 K
done
clear
B)
250 K
done
clear
C)
750 K
done
clear
D)
125 K
done
clear
View Answer play_arrow
question_answer 26) The average kinetic energy per molecule of the gas is
A)
\[\frac{1}{2}kT\]
done
clear
B)
\[\frac{3}{2}kT\]
done
clear
C)
\[\frac{1}{2}RT\]
done
clear
D)
\[\frac{3}{2}RT\]
done
clear
View Answer play_arrow
question_answer 27) If the temperature of gas increases from\[27{}^\circ C\]to \[927{}^\circ C,\]the KE will be
A)
double
done
clear
B)
half
done
clear
C)
one-fourth
done
clear
D)
four times
done
clear
View Answer play_arrow
question_answer 28) Choose the correct relation. (where p = pressure, E = kinetic energy of gas)
A)
\[p=\frac{3}{2}E\]
done
clear
B)
\[p=\frac{2}{3}E\]
done
clear
C)
\[p=\frac{1}{2}E\]
done
clear
D)
\[p=\,2E\]
done
clear
View Answer play_arrow
question_answer 29) For a perfectly black body, its absorption coefficient will be
A)
1
done
clear
B)
0
done
clear
C)
between 0 and 1
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 30) The heat given to diatomic gas at constant pressure, the how many quantity will be change in internal energy?
A)
5/7
done
clear
B)
3/5
done
clear
C)
2/5
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 31) The relation between velocities of\[{{O}_{2}}\]and\[{{H}_{2}}\]
A)
\[v{{H}_{2}}=4{{v}_{O2}}\]
done
clear
B)
\[{{v}_{O2}}=4{{V}_{H2}}\]
done
clear
C)
\[{{v}_{O2}}=2{{v}_{H2}}\]
done
clear
D)
\[{{v}_{H2}}=2{{v}_{O2}}\]
done
clear
View Answer play_arrow
question_answer 32) The quality of metal of cooking vessel
A)
less specific heat and more conduction
done
clear
B)
more specific heat and less conduction
done
clear
C)
more specific heat and more conduction
done
clear
D)
less specific heat and less conduction
done
clear
View Answer play_arrow
question_answer 33) Volume of gas becomes four times if
A)
temperature becomes four times at constant pressure
done
clear
B)
temperature becomes one fourth at constant pressure
done
clear
C)
temperature becomes two times at constant pressure
done
clear
D)
temperature becomes half at constant pressure
done
clear
View Answer play_arrow
question_answer 34) A source of frequency 150 Hz is moving in the direction of a person with a velocity of 110 m/s. The frequency heard by the person will be (speed of sound medium = 330 m/s)
A)
225 Hz
done
clear
B)
250 Hz
done
clear
C)
495 Hz
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 35) To increase the frequency from 100 Hz to 400 Hz, the tension of string will be
A)
4 times
done
clear
B)
16 times
done
clear
C)
20 times
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 36) A big ball of mass M, moving with velocity u strikes a small ball of mass m, which is at rest. Finally small ball obtains velocity u and big ball v. Then what is the value of v?
A)
\[\frac{M-m}{M}u\]
done
clear
B)
\[\frac{m}{M+m}u\]
done
clear
C)
\[\frac{2m}{M+m}u\]
done
clear
D)
\[\frac{M}{M+m}u\]
done
clear
View Answer play_arrow
question_answer 37) Twenty seven drops of water of the same size are equally potential V. They are then united to form a bigger drop. By what factor will the electrical potential changes?
A)
9V
done
clear
B)
3V
done
clear
C)
27V
done
clear
D)
\[\frac{1}{3}V\]
done
clear
View Answer play_arrow
question_answer 38) The power factor of L-C- R circuit at resonance is
A)
zero
done
clear
B)
1
done
clear
C)
0.5
done
clear
D)
0.766
done
clear
View Answer play_arrow
question_answer 39) An alternating current of frequency / is flowing in a circuit containing a resistance R and choke L in series. The impedance of this circuit is
A)
\[\sqrt{{{R}^{2}}+\,{{(2\pi fL)}^{2}}}\]
done
clear
B)
\[\sqrt{{{R}^{2}}+\,{{(2\pi {{f}^{2}})}^{2}}}\]
done
clear
C)
\[\sqrt{{{R}^{2}}+\,L\pi {{f}^{2}}}\]
done
clear
D)
\[{{R}^{2}}-\,{{(2\pi {{f}^{2}})}^{2}}\]
done
clear
View Answer play_arrow
question_answer 40) V rms = 220 V, then the peak value of voltage is
A)
311 V
done
clear
B)
281 V
done
clear
C)
200 V
done
clear
D)
181 V
done
clear
View Answer play_arrow
question_answer 41) The magnetic moment of coil is M and area is A. Then value of current will be
A)
\[\frac{2M}{A}\]
done
clear
B)
\[\frac{M}{A}\]
done
clear
C)
\[\frac{A}{M}\]
done
clear
D)
\[\frac{M}{2A}\]
done
clear
View Answer play_arrow
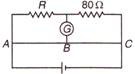
question_answer 42)
In the circuit shown below, the current of 2A is flowing, the impedance of circuit will be
A)
170 \[\Omega \]
done
clear
B)
70 \[\Omega \]
done
clear
C)
130 \[\Omega \]
done
clear
D)
120 \[\Omega \]
done
clear
View Answer play_arrow
question_answer 43) In AC circuit\[E={{E}_{0}}sin\]at and\[I={{I}_{0}}sin\]\[\left( \omega t-\frac{\pi }{2} \right)\]then power dissipated will be
A)
zero
done
clear
B)
\[\frac{{{E}_{0}}{{I}_{0}}}{2}\]
done
clear
C)
\[{{E}_{rms}}{{I}_{rms}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 44)
If\[{{C}_{air}}=10\]\[\mu \]F, then effective capacity will be
A)
30\[\mu \]F
done
clear
B)
15\[\mu \]F
done
clear
C)
3 \[\mu \]F
done
clear
D)
10 \[\mu \]F
done
clear
View Answer play_arrow
question_answer 45) A voltmeter has a resistance of G ohm and range V volt. The value of resistance used in series to convert it into a voltmeter of range nV volt is
A)
nG
done
clear
B)
(n-1)G
done
clear
C)
\[\frac{G}{n}\]
done
clear
D)
\[\frac{G}{(n-1)}\]
done
clear
View Answer play_arrow
question_answer 46) The magnetic moment of current loop is\[2.1\times {{10}^{-25}}\]\[A-{{m}^{2}}\]. The magnetic field at distance 1 \[\overset{o}{\mathop{\text{A}}}\,\]from centre on axis of loop will be
A)
\[4.2\times {{10}^{2}}Wb/{{m}^{2}}\]
done
clear
B)
\[4.2\times {{10}^{-3}}Wb/{{m}^{2}}\]
done
clear
C)
\[4.2\times {{10}^{-4~}}Wb/{{m}^{2}}\]
done
clear
D)
\[4.2\times {{10}^{-5}}Wb/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 47) The intensity of electric field at a point on the axis of an electric dipole depends on the distance r from the centre of the dipole as
A)
\[E\propto \frac{1}{{{r}^{2}}}\]
done
clear
B)
\[E\,\propto \frac{1}{{{r}^{3}}}\]
done
clear
C)
\[E\propto \,{{r}^{2}}\]
done
clear
D)
\[E\propto \,\frac{1}{{{r}^{4}}}\]
done
clear
View Answer play_arrow
question_answer 48) How many electrons are in 1 coulomb charge
A)
\[6.25\times {{10}^{18}}\]
done
clear
B)
\[1.6\times {{10}^{19}}\]
done
clear
C)
100
done
clear
D)
\[6.25\times {{10}^{12}}\]
done
clear
View Answer play_arrow
question_answer 49) When charge q enters perpendicular in magnetic field B, then frequency will be
A)
\[\frac{2\pi m}{qB}\]
done
clear
B)
\[\frac{qB}{2\pi m}\]
done
clear
C)
\[\frac{qB}{\pi m}\]
done
clear
D)
\[\frac{\pi m}{qB}\]
done
clear
View Answer play_arrow
question_answer 50) The resistance of ammeter is 99ft. The current of ammeter is 10% of main current, then the resistance of shunt will be
A)
11\[\Omega \]
done
clear
B)
22\[\Omega \]
done
clear
C)
99\[\Omega \]
done
clear
D)
33\[\Omega \]
done
clear
View Answer play_arrow
question_answer 51) \[E=2\text{ }V/m\]at distance 60 cm from the charge, then charge will be
A)
\[8\times {{10}^{-11}}C\]
done
clear
B)
\[8\times {{10}^{11}}C\]
done
clear
C)
\[4\times {{10}^{11}}C\]
done
clear
D)
\[4\times {{10}^{-11}}C\]
done
clear
View Answer play_arrow
question_answer 52)
AB is a wire of uniform resistance. The galvanometer G shows no current when the length AB=20 cm and BC =80 cm. The resistance R is equal to
A)
20\[\Omega \]
done
clear
B)
80\[\Omega \]
done
clear
C)
10\[\Omega \]
done
clear
D)
40\[\Omega \]
done
clear
View Answer play_arrow
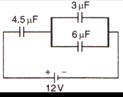
question_answer 53)
The potential difference on capacitor of 4.5 \[\mu \]F
A)
8 V
done
clear
B)
4 V
done
clear
C)
2 V
done
clear
D)
6 V
done
clear
View Answer play_arrow
question_answer 54) If in a transformer, the Lechlanche cell is connected across the primary, then in the secondary coil
A)
emf will be induced
done
clear
B)
any emf will not be induced
done
clear
C)
emf will be increase
done
clear
D)
emf will be decrease
done
clear
View Answer play_arrow
question_answer 55) The unit of inductance
A)
\[\frac{volt\,\times \,\sec ond}{ampere}\]
done
clear
B)
\[\frac{volt\,\times \,ampere}{\sec ond}\]
done
clear
C)
\[\frac{volt}{ampere}\]
done
clear
D)
\[\frac{volt\,\times \,{{(ampere)}^{2}}}{\sec ond}\]
done
clear
View Answer play_arrow
question_answer 56) Where does the intensity of electric field become zero in hollow sphere?
A)
At external points
done
clear
B)
At outer surface
done
clear
C)
At internal points
done
clear
D)
At inside and outside
done
clear
View Answer play_arrow
question_answer 57) In Young's double slit experiment, the screen is kept at a distance 2m from source. The distance between light wavelength of 6000\[\overset{o}{\mathop{\text{A}}}\,\]and phase mode source is 4 mm. The band width will be
A)
0.2 m
done
clear
B)
2 cm
done
clear
C)
20 cm
done
clear
D)
0.03 cm
done
clear
View Answer play_arrow
question_answer 58) The mean distance between the atoms of metal is \[3\times {{10}^{-10}}m\]and interatomic force constant for metal is\[3.6\times {{10}^{-9}}N/A,\]then Young's modulus of metal is
A)
\[1.2\times {{10}^{11}}N/{{m}^{2}}\]
done
clear
B)
\[4.2\times {{10}^{11}}N/{{m}^{2}}\]
done
clear
C)
\[10.8\times {{10}^{-19}}N/{{m}^{2}}\]
done
clear
D)
\[2.4\times {{10}^{10}}N/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 59) If light polarized by the reflection then the angle between reflected wave and refracted wave will be
A)
\[\pi \]
done
clear
B)
\[\pi /2\]
done
clear
C)
\[2\pi \]
done
clear
D)
\[\pi /4\]
done
clear
View Answer play_arrow
question_answer 60) Condition of diffraction is
A)
\[\frac{a}{\lambda }=1\]
done
clear
B)
\[\frac{a}{\lambda }>>1\]
done
clear
C)
\[\frac{a}{\lambda }<<1\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 61) If photoelectric effect experiment, the intensity of incident ray increases, then the correct statement is
A)
the kinetic energy of emitted electron will be maximum
done
clear
B)
work function will be unchanged
done
clear
C)
stopping potential will be increases
done
clear
D)
the kinetic energy of emitted electron will be become less
done
clear
View Answer play_arrow
question_answer 62) During the \[{{\beta }^{-}}\] decay
A)
neutron, converts into proton
done
clear
B)
proton converts into neutron
done
clear
C)
neutron-proton ratio increases
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 63) In depletion layer, there are
A)
immobile ions
done
clear
B)
free charge carries
done
clear
C)
charge carrier and immobile ions
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 64) The relation among the forbidden energy band gap in conductors, semiconductors and insulators is
A)
\[\Delta E{{g}_{c}}\]>\[\Delta E{{g}_{s}}\]>\[\Delta E{{g}_{i}}\]
done
clear
B)
\[\Delta E{{g}_{i}}\]>\[\Delta E{{g}_{s}}\]>\[\Delta E{{g}_{c}}\]
done
clear
C)
\[\Delta E{{g}_{c}}\]>\[\Delta E{{g}_{c}}\]>\[\Delta E{{g}_{s}}\]
done
clear
D)
\[\Delta E{{g}_{s}}\]>\[\Delta E{{g}_{c}}\]>\[\Delta E{{g}_{s}}\]
done
clear
View Answer play_arrow
question_answer 65) By increasing the temperature of semi- conductors, its resistance
A)
increase
done
clear
B)
decrease
done
clear
C)
remain constant
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 66)
The value of current in the circuit
A)
zero
done
clear
B)
1 A
done
clear
C)
0.1 A
done
clear
D)
0.2 A
done
clear
View Answer play_arrow
question_answer 67) Good nuclear fuel is
A)
\[{{U}^{238}}\]
done
clear
B)
\[P{{u}^{238}}\]
done
clear
C)
\[T{{h}^{234}}\]
done
clear
D)
\[{{U}^{235}}\]
done
clear
View Answer play_arrow
question_answer 68) The binding energy of K-shell of electron is\[-40000\]eV. Applied the potential of 60000 V in Coolidge tube, X-rays will be
A)
continuous X-ray
done
clear
B)
white X-rays
done
clear
C)
continuous and all characteristics X-rays
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 69) The typical ionization energy of a donor in silicon is
A)
10.0 eV
done
clear
B)
1.0 eV
done
clear
C)
0.1 eV
done
clear
D)
0.001 Ev
done
clear
View Answer play_arrow
question_answer 70) If the threshold frequency of sodium metal is 6800 A, then work function will be
A)
1.8 eV
done
clear
B)
2.5 eV
done
clear
C)
2.1 eV
done
clear
D)
1.4 eV
done
clear
View Answer play_arrow
question_answer 71) If elements with quantum number n > 4 are not in the nature, the number of possible elements is
A)
60
done
clear
B)
32
done
clear
C)
4
done
clear
D)
64
done
clear
View Answer play_arrow
question_answer 72) If capillary experiment is performed in vacuum, then for a liquid there
A)
it will rise
done
clear
B)
will remain same
done
clear
C)
it will fall
done
clear
D)
rise to the top
done
clear
View Answer play_arrow
question_answer 73) The reason of Avalanche breakdown is
A)
collision of minority charge carriers
done
clear
B)
increasing of depletion layer
done
clear
C)
decreasing of depletion layer
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 74) Which of the following relations are correct?
A)
\[E=\,\frac{hc}{\lambda }\]
done
clear
B)
\[E=\,\frac{1}{2}m{{u}^{2}}\]
done
clear
C)
\[P=\,\frac{E}{2v}\]
done
clear
D)
\[E=\,\frac{1}{2}m{{c}^{2}}\]
done
clear
View Answer play_arrow
question_answer 75) Ionic potential for second electron of helium is
A)
13.6 eV
done
clear
B)
27.2 eV
done
clear
C)
54.4 eV
done
clear
D)
100 eV
done
clear
View Answer play_arrow
question_answer 76) Which of the following relations are correct for characteristics X-rays?
A)
\[\lambda \,\alpha \,\frac{1}{{{Z}^{2}}}\]
done
clear
B)
\[\lambda \,\alpha \,{{Z}^{2}}\]
done
clear
C)
\[\lambda \times Z\]
done
clear
D)
\[1\,\alpha \frac{1}{V}\]
done
clear
View Answer play_arrow
question_answer 77) The beam of 1 \[\mu \]A of proton whose cross-section area is\[0.5\text{ }m{{m}^{2}}\]and velocity is\[3\times {{10}^{4}}m/s,\]then charge density of beam
A)
\[6.6\,\times \,{{10}^{-4}}C/{{m}^{3}}\]
done
clear
B)
\[6.6\,\times \,{{10}^{-5}}C/{{m}^{3}}\]
done
clear
C)
\[6.6\,\times \,{{10}^{-6}}C/{{m}^{3}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 78) The correct relation for elastic potential energy is
A)
energy density = \[\frac{1}{2}\]\[\times \]strain\[\times \]stress
done
clear
B)
energy density =\[{{(strain)}^{2}}\]\[\times \] volume
done
clear
C)
energy density = strain \[\times \] volume
done
clear
D)
energy density = \[{{(strain)}^{2}}\]\[\times \] volume
done
clear
View Answer play_arrow
question_answer 79) The ratio of the longest to shortest wavelengths in Brackett series of hydrogen spectrum is
A)
\[\frac{25}{9}\]
done
clear
B)
\[\frac{17}{6}\]
done
clear
C)
\[\frac{9}{5}\]
done
clear
D)
\[\frac{4}{3}\]
done
clear
View Answer play_arrow
question_answer 80) An Indian rubber cord L metre long and area of cross-section A metre2 is suspended vertically. Density of rubber is\[D\text{ }kg/{{m}^{2}}\]and Young's modulus of rubber is\[E\text{ }n/{{m}^{2}}\]. If the wire extends by 1 m under its own height; then extension in length / is
A)
\[{{L}^{2}}Dg/E\]
done
clear
B)
\[{{L}^{2}}Dg/2E\]
done
clear
C)
\[{{L}^{2}}Dg/4E\]
done
clear
D)
\[L\]
done
clear
View Answer play_arrow
question_answer 81) The nature of electromagnetic waves is
A)
transverse
done
clear
B)
logitudinal
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 82) Radius of a soap bubble is increased from R to 2R, work done is this process in terms of surface tension is
A)
\[24\pi {{R}^{2}}T\]
done
clear
B)
\[12T\pi {{r}^{2}}\]
done
clear
C)
\[6T\pi {{r}^{2}}\]
done
clear
D)
\[4T\pi {{r}^{2}}\]
done
clear
View Answer play_arrow
question_answer 83) Light is incident normally on diffraction grating through which the first order, diffraction is seen\[32{}^\circ \]. The second order diffraction will be seen at
A)
\[48{}^\circ \]
done
clear
B)
\[64{}^\circ \]
done
clear
C)
\[80{}^\circ \]
done
clear
D)
There is no second order diffraction in this case
done
clear
View Answer play_arrow
question_answer 84) If liquid level fall in a capillary, then radius of curvature in capillary tube will
A)
increase
done
clear
B)
decrease
done
clear
C)
unchanged
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 85) The relative velocity of two consecutive layer is 8 cm/s. If the perpendicular distance between the layers is 0.1 m, then the velocity gradient will be
A)
\[50\text{ }{{s}^{-1}}\]
done
clear
B)
\[\text{60 }{{s}^{-1}}\]
done
clear
C)
\[\text{75 }{{s}^{-1}}\]
done
clear
D)
\[\text{80 }{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 86) The pressure below in surface area of water in the capillary tube will be
A)
equal to atmospheric pressure
done
clear
B)
equal to above pressure
done
clear
C)
less than above pressure
done
clear
D)
more than above pressure
done
clear
View Answer play_arrow
question_answer 87) The ratio of intensities of bright and dark fringes are given by 4:1. The ratio of the amplitudes of the two waves is
A)
1 : 1
done
clear
B)
3 : 1
done
clear
C)
1 : 3
done
clear
D)
1 : 9
done
clear
View Answer play_arrow
question_answer 88) An object is at a temperature of\[400{}^\circ C\]. At what temperature would it radiate energy twice as fast? The temperature of the surroundings may be assumed to be negligible
A)
\[200{}^\circ C\]
done
clear
B)
200 K
done
clear
C)
\[800{}^\circ C\]
done
clear
D)
800 K
done
clear
View Answer play_arrow
question_answer 89) tress to strain ratio is equivalent to
A)
Modulus of elasticity
done
clear
B)
Poisson's ratio
done
clear
C)
Reynold number
done
clear
D)
Fund number
done
clear
View Answer play_arrow
question_answer 90) In an isothermal reversible expansion, if the volume of 96 g of oxygen at\[27{}^\circ C\]is increased from 70 litre to 140 litre, then the work done by the gas will be
A)
\[300\text{ }R\text{ }lo{{g}_{10}}2\]
done
clear
B)
\[81\text{ }R\text{ }lo{{g}_{e}}2\]
done
clear
C)
\[900\text{ }R\text{ }lo{{g}_{10}}2\]
done
clear
D)
\[2.3\times 900\text{ }R\text{ }lo{{g}_{10}}2\]
done
clear
View Answer play_arrow
question_answer 91) To get the maximum current from a parallel combination of n identical cells each of internal resistance r in an external resistance R when
A)
R>>r
done
clear
B)
R<<r
done
clear
C)
R = r
done
clear
D)
None of these
done
clear
View Answer play_arrow
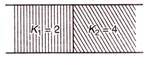
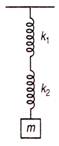
question_answer 92)
A mass M is suspended by two springs of force constants\[{{k}_{1}}\]and\[{{k}_{2}}\]respectively as shown in the diagram. The total elongation (stretch) of the two springs is
A)
\[\left( \frac{{{k}_{1}}+{{k}_{2}}}{{{k}_{1}}{{k}_{2}}} \right)\]
done
clear
B)
\[\frac{2({{k}_{1}}+{{k}_{2}})}{{{k}_{1}}{{k}_{2}}}mg\]
done
clear
C)
\[\frac{2({{k}_{1}}+{{k}_{2}})}{{{k}_{1}}{{k}_{2}}}mg\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 93) A potentiometer has uniform potential gradient. The specific resistance of the material of the potentiometer wire is \[40\times {{10}^{-8}}m\] and current passing through it is 15 A, cross-section area of the wire is\[18\times {{10}^{-6}}{{m}^{2}}\]. The potential gradient along the potentiometer wire is
A)
25 V/m
done
clear
B)
2.5 V/cm
done
clear
C)
2.5 V/m
done
clear
D)
0.33 V/m
done
clear
View Answer play_arrow
question_answer 94) Work done in splitting a drop of water of 1 mm radius into \[{{10}^{6}}\]droplets is (surface tension of water\[=72\times {{10}^{-3}}J/{{m}^{2}}\])
A)
\[89.5\times {{10}^{-5}}J\]
done
clear
B)
0.895 J
done
clear
C)
\[895\times {{10}^{-5}}J\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 95) The size of magnet to generate the radial magnetic field in moving coil galvanometer will be
A)
concave
done
clear
B)
horse shoe magnet
done
clear
C)
convex
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 96) Transverse elastic wave can passes
A)
in gas and solid
done
clear
B)
in solid but not in gas
done
clear
C)
in gas but not in solid
done
clear
D)
None of the above
done
clear
View Answer play_arrow
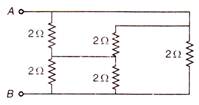
question_answer 97)
The equivalent resistance between the points A and B is
A)
1\[\Omega \]
done
clear
B)
2\[\Omega \]
done
clear
C)
3\[\Omega \]
done
clear
D)
4\[\Omega \]
done
clear
View Answer play_arrow
question_answer 98) A potentiometer has uniform potential gradient across it. Two cells connected in series (i) to support each other and (ii) to oppose each other are balanced over 6 m and 2 m respectively on the potentiometer wire. The emf's of the cells are in the ratio of
A)
1 : 2
done
clear
B)
1 : 1
done
clear
C)
3 : 1
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 99) A Carnot engine working between 300 K and 600 K has work output of 800 j per cycle. What is amount of heat energy supplied to the engine from source, per cycle?
A)
1800 J
done
clear
B)
1000 J
done
clear
C)
2000 J
done
clear
D)
1600 J
done
clear
View Answer play_arrow
question_answer 100) The ideal gas expands in such a manner that its pressure and volume can be related by equation\[p{{V}^{2}}=\]constant. During this process, the gas is
A)
heated
done
clear
B)
cooled
done
clear
C)
neither heated nor cooled
done
clear
D)
first heated and then cooled
done
clear
View Answer play_arrow
question_answer 101) Structure of\[{{C}_{2}}{{H}_{2}}\] is
A)
linear
done
clear
B)
trigonal planar
done
clear
C)
pyramidal
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 102) In \[CsCl\]crystal, if\[C{{s}^{+}}\] ion is surrounded from \[8C{{l}^{-}}\]ions and \[C{{l}^{-}}\]ion from\[C{{s}^{+}}\] ion then coordination number will be
A)
4
done
clear
B)
6
done
clear
C)
8
done
clear
D)
12
done
clear
View Answer play_arrow
question_answer 103) Lattice energy is
A)
energy used in the formation of 1 mole of solid crystal
done
clear
B)
energy released in the formation of 1 mole of solid crystal
done
clear
C)
energy released in the formation of 1 g of solid crystal
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 104) If the value of \[{{K}_{a}}\] for acid is (I) \[1.36\times {{10}^{-5}}\] (II) \[125\times {{10}^{-5}}\] (III) \[260\times {{10}^{-5}}\] (IV) \[67\times {{10}^{-5}}\] then the strongest acid will be
A)
I
done
clear
B)
II
done
clear
C)
III
done
clear
D)
IV
done
clear
View Answer play_arrow
question_answer 105) A solution obtained on mixing 0.1 N, 50 mL \[HCl\]and 0.1 N, 50 mL\[NaOH\]. The value of pH of the solution will be
A)
4
done
clear
B)
6
done
clear
C)
8
done
clear
D)
7
done
clear
View Answer play_arrow
question_answer 106) The oxidation number of Cr in\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]is
A)
6
done
clear
B)
4
done
clear
C)
7
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 107) Correct order of bond length is
A)
\[HC\equiv CH>{{H}_{2}}C=C{{H}_{2}}>{{H}_{3}}C-C{{H}_{3}}\]
done
clear
B)
\[{{H}_{2}}C=C{{H}_{2}}>HC\equiv CH>{{H}_{3}}C-C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}-C{{H}_{3}}>C{{H}_{2}}=C{{H}_{2}}>HC\equiv CH\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{3}}>HC\equiv CH>{{H}_{2}}C=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 108) \[A+BC+D\] If the concentration of A is doubled then the rate of reaction will
A)
remain unchanged
done
clear
B)
become four times
done
clear
C)
become two times
done
clear
D)
become quarter
done
clear
View Answer play_arrow
question_answer 109) For endothermic reaction, on increasing temperature the value of\[{{K}_{eq}}\]will
A)
increase
done
clear
B)
decrease
done
clear
C)
approximately remain unchanged
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 110) The type of hybridization in\[C{{H}_{2}}=C=C{{H}_{2}}\]is
A)
only \[s{{p}^{2}}\]
done
clear
B)
only\[sp\]
done
clear
C)
\[sp\]and\[s{{p}^{2}}\]
done
clear
D)
\[s{{p}^{2}}\]and\[s{{p}^{3}}\]
done
clear
View Answer play_arrow
question_answer 111) Which of the following has the maximum electronegativity?
A)
F
done
clear
B)
\[Cl\]
done
clear
C)
N
done
clear
D)
\[O\]
done
clear
View Answer play_arrow
question_answer 112) Which of the following effect will observe, on passing light from colloidal solution?
A)
Electrophoresis
done
clear
B)
Tyndall effect
done
clear
C)
Electro osmosis
done
clear
D)
Coagulation
done
clear
View Answer play_arrow
question_answer 113) The order of the following reaction is three, \[6KI+2FeC{{l}_{3}}\xrightarrow[{}]{{}}2FeI+6KCl+2{{I}_{2}}\] What will be the molecularity of this reaction?
A)
3
done
clear
B)
8
done
clear
C)
\[-1\]
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 114) Which of the following indicator will be used in the following reaction? \[KMn{{O}_{4}}+F{{e}^{2+}}\xrightarrow[{}]{{}}\]Product
A)
Methyl red
done
clear
B)
Phenolphthalein
done
clear
C)
Phenol red
done
clear
D)
Self indicator
done
clear
View Answer play_arrow
question_answer 115) At constant temperature, the relationship between osmotic pressure and volume will be
A)
\[p\propto V\]
done
clear
B)
\[p\propto \frac{1}{V}\]
done
clear
C)
\[p\propto \sqrt{V}\]
done
clear
D)
\[p\]
done
clear
View Answer play_arrow
question_answer 116) The pH of a solution is 4.0. On adding phenolphthalein in this solution, the colour of solution will be
A)
pink
done
clear
B)
yellow
done
clear
C)
red
done
clear
D)
colourless
done
clear
View Answer play_arrow
question_answer 117) The nature of\[{{H}_{2}}{{O}_{2}}\] is
A)
weak basic
done
clear
B)
weak acidic
done
clear
C)
strong acidic
done
clear
D)
strong basic
done
clear
View Answer play_arrow
question_answer 118) The product will be obtained in the following reaction \[{{K}_{4}}[Fe{{(CN)}_{6}}]+{{O}_{3}}\xrightarrow[{}]{{{H}_{2}}O}\]Product
A)
\[F{{e}^{2+}}\]ion
done
clear
B)
\[{{K}_{3}}[Fe{{(CN)}_{6}}]\]
done
clear
C)
\[C{{N}^{-}}\]ion
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 119) According to Pauli exclusion principle, any two electrons in an atom cannot have identical set of ....... quantum number.
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 120) Magnetic moment of\[C{{u}^{+}}\]ion is
A)
1.73
done
clear
B)
0
done
clear
C)
2.83
done
clear
D)
3.95
done
clear
View Answer play_arrow
question_answer 121) Which of the following has\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},\] \[3{{s}^{2}}3{{p}^{6}},3{{d}^{10}}4{{s}^{2}}\]electronic configuration?
A)
Zn
done
clear
B)
\[Cu\]
done
clear
C)
\[Co\]
done
clear
D)
\[Ni\]
done
clear
View Answer play_arrow
question_answer 122) Which of the following has zero bond order?
A)
\[He_{2}^{+}\]
done
clear
B)
\[H{{e}_{2}}\]
done
clear
C)
\[H_{2}^{+}\]
done
clear
D)
\[H_{2}^{-}\]
done
clear
View Answer play_arrow
question_answer 123) An element has the following characters (1) atomic number : 31 (2) with\[HN{{O}_{2}}:\]no reaction (3) reaction with\[{{O}_{2}}:\]no reaction (4) on heating form a layer of oxide (5) used in transistor then the element will be
A)
Ga
done
clear
B)
\[In\]
done
clear
C)
Br
done
clear
D)
\[Mg\]
done
clear
View Answer play_arrow
question_answer 124) Element\[Ca,Sr,Ba\]show colours in Bunsen's burner flame due to
A)
low ionization potential value
done
clear
B)
emission and absorption of energy by electron
done
clear
C)
high ionization potential value
done
clear
D)
transfer of energy
done
clear
View Answer play_arrow
question_answer 125) Decreasing order of radius of hydrated ions of \[Li,Na,K,Rb\]and Cs is
A)
\[Li>Na>K>Rb>Cs\]
done
clear
B)
\[Cs>Rb>K>Na>Li\]
done
clear
C)
\[Cs>Rb>K>Li>Na\]
done
clear
D)
\[Cs>K>Rb>Na>Li\]
done
clear
View Answer play_arrow
question_answer 126) If the frequency of sodium ion is\[5.09\times {{10}^{14}}{{s}^{-1}}\]then wavelength will be
A)
5.89nm
done
clear
B)
589 nm
done
clear
C)
58.9 nm
done
clear
D)
5890 nm
done
clear
View Answer play_arrow
question_answer 127) Electron deficient compound is
A)
\[PC{{l}_{3}}\]
done
clear
B)
\[BC{{l}_{3}}\]
done
clear
C)
\[ICl\]
done
clear
D)
\[PC{{l}_{5}}\]
done
clear
View Answer play_arrow
question_answer 128) In the following reaction, \[N{{H}_{3}}+B{{F}_{3}}\xrightarrow[{}]{{}}N{{H}_{3}}\to B{{F}_{3}}\] which behaves like Lewis base?
A)
\[N{{H}_{3}}\]
done
clear
B)
\[B{{F}_{3}}\]
done
clear
C)
\[N{{H}_{3}}\to B{{F}_{3}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 129) For\[{{H}_{2}}O\]and\[{{D}_{2}}O,\]the correct statement is
A)
Both have same boiling point
done
clear
B)
Surface tension of\[{{D}_{2}}O\]is less than\[{{H}_{2}}O\]
done
clear
C)
Viscosity of\[{{H}_{2}}O\]is greater than \[{{D}_{2}}O\]
done
clear
D)
Solubility of\[{{D}_{2}}O\]is more in ionic compound
done
clear
View Answer play_arrow
question_answer 130) When\[BaS\]reacts with\[{{D}_{2}}O\] then products obtained
A)
\[BaO+{{D}_{2}}S\]
done
clear
B)
\[Ba{{(OD)}_{2}}+{{D}_{2}}S\]
done
clear
C)
\[BaS{{O}_{4}}.{{D}_{2}}O+S\]
done
clear
D)
\[Ba{{O}_{2}}+{{D}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 131) In the reaction,\[4LiH+AlC{{l}_{3}}\xrightarrow[{}]{Ether}\]Product. The product is
A)
\[LiCl\]
done
clear
B)
\[L{{i}_{2}}O\]
done
clear
C)
\[LiAl{{H}_{4}}\]
done
clear
D)
\[Al{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 132) If mass of\[C{{O}_{2}}\]molecule is 44u and Avogadro's number is\[6.023\times {{10}^{23}}\]then mass of one molecule in kg is
A)
\[16\times {{10}^{-28}}kg\]
done
clear
B)
\[2.16\times {{10}^{-22}}kg\]
done
clear
C)
\[3.46\times {{10}^{-28}}kg\]
done
clear
D)
\[7.3\times {{10}^{-26}}kg\]
done
clear
View Answer play_arrow
question_answer 133) Molecular weight of a compound is 96.5 and has the following characters. (1) poisonous (2) isodiapher (3) finds in cis and trans form (4) hypnotic then the compound is
A)
\[{{C}_{2}}HC{{l}_{3}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{2}}C{{l}_{2}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{3}}Cl\]
done
clear
D)
\[{{C}_{2}}C{{l}_{4}}\]
done
clear
View Answer play_arrow
question_answer 134) Presence of three unpaired electrons in p-orbital of phosphorus can be explained by
A)
Aufbau rule
done
clear
B)
Hund's rule
done
clear
C)
Faults rule
done
clear
D)
\[(n+7)\]rule
done
clear
View Answer play_arrow
question_answer 135) Colloidal solution is not formed by
A)
precipitation
done
clear
B)
peptisation
done
clear
C)
double decomposition
done
clear
D)
mechanical decomposition
done
clear
View Answer play_arrow
question_answer 136) Formula of bauxite is
A)
\[A{{l}_{2}}{{O}_{3}}.2{{H}_{2}}O\]
done
clear
B)
\[A{{l}_{2}}{{O}_{3}}.{{H}_{2}}O\]
done
clear
C)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
D)
\[A{{l}_{2}}{{O}_{3}}.3{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 137) Decrease in oxidation number is called
A)
oxidation
done
clear
B)
reduction
done
clear
C)
redox reaction
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 138) For a buffer solution of\[C{{H}_{3}}COOH\]and \[C{{H}_{3}}COONa\]if\[[C{{H}_{3}}COOH],[C{{H}_{3}}COONa]=1\] and\[p{{K}_{a}}=4.74\]the pH value of the solution will be
A)
5.74
done
clear
B)
6.74
done
clear
C)
3.74
done
clear
D)
4.74
done
clear
View Answer play_arrow
question_answer 139) If\[pH=8\]solution is basic, then the nature of this solution will be corresponding to that solution whose\[pH=12\]
A)
less basic
done
clear
B)
more basic
done
clear
C)
equal
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 140) CNG is
A)
\[C{{H}_{4}}\] (84%) + propane + butane + higher alkane
done
clear
B)
\[C{{H}_{4}}\](33%) + ethane (33%) + butane (33%)
done
clear
C)
benzene (10%) + petrol (90%)
done
clear
D)
\[C{{H}_{4}}\] (10%) + LPG (90%)
done
clear
View Answer play_arrow
question_answer 141) Which of the following compound is strongly polar and has high dipole moment?
A)
\[CHC{{l}_{3}}\]
done
clear
B)
\[C{{H}_{2}}C{{l}_{2}}\]
done
clear
C)
\[CC{{l}_{4}}\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 142) If the hybridization in\[{{C}_{2}}{{H}_{2}}\]is sp, then what will be the bond angle?
A)
\[180{}^\circ \]
done
clear
B)
\[120{}^\circ \]
done
clear
C)
\[150{}^\circ \]
done
clear
D)
\[90{}^\circ \]
done
clear
View Answer play_arrow
question_answer 143) Isoelectronic for\[{{K}^{+}}\] is
A)
\[Ar\]
done
clear
B)
\[Cl\]
done
clear
C)
S
done
clear
D)
\[Ca\]
done
clear
View Answer play_arrow
question_answer 144) When \[{{H}_{2}}\]reacts with\[C{{l}_{2}}\] in presence of sunlight to form \[HCl\]then order of reaction will be (unit of\[K=mol\,{{L}^{-1}}{{S}^{-1}}\])
A)
2
done
clear
B)
3
done
clear
C)
0
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 145) Correct order of ionization potential is
A)
\[B>Be\]
done
clear
B)
\[Be>B\]
done
clear
C)
\[Be=B\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 146) According to the reaction, \[Cu+N{{H}_{3}}\xrightarrow[{}]{Air}\] Product;, The product is
A)
\[C{{u}^{2+}}\]
done
clear
B)
\[C{{u}^{2+}}.NO\]
done
clear
C)
\[[Cu{{(N{{H}_{3}})}_{4}}]{{(OH)}_{2}}\]
done
clear
D)
\[Cu{{(OH)}_{2}}\]
done
clear
View Answer play_arrow
question_answer 147) Main ore of an iron is
A)
\[F{{e}_{3}}{{O}_{4}}\]
done
clear
B)
\[FeC{{O}_{3}}\]
done
clear
C)
\[F{{e}_{2}}{{O}_{3}}\]
done
clear
D)
\[Fe{{S}_{2}}\]
done
clear
View Answer play_arrow
question_answer 148) Diagonal relationship shows
A)
\[Ca\]and Ba
done
clear
B)
Be and Mg
done
clear
C)
\[Na\]and K
done
clear
D)
Li and Be
done
clear
View Answer play_arrow
question_answer 149) Number of crand n bonds in\[A-N=N-B\]
A)
\[3\sigma ,1\pi \]
done
clear
B)
\[2\sigma ,0\pi \]
done
clear
C)
\[2\sigma ,2\pi \]
done
clear
D)
\[4\sigma ,0\pi \]
done
clear
View Answer play_arrow
question_answer 150) Which of the following compound has zero dipole moment?
A)
\[C{{O}_{2}}\]
done
clear
B)
\[S{{O}_{2}}\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 151) If the value of velocity indices are 2 a, 3b and 1c then the value of Miller's indices will be
A)
3, 2, 6
done
clear
B)
2, 3, 1
done
clear
C)
1, 2, 3
done
clear
D)
1, 1, 1
done
clear
View Answer play_arrow
question_answer 152) Radius of\[_{6}{{C}^{12}}\]nucleus will be
A)
\[3.2\times {{10}^{-13}}cm\]
done
clear
B)
\[1.6\times {{10}^{-13}}cm\]
done
clear
C)
\[4.8\times {{10}^{-13}}cm\]
done
clear
D)
\[6.4\times {{10}^{-3}}cm\]
done
clear
View Answer play_arrow
question_answer 153) Electron affinity of nitrogen is
A)
0.2
done
clear
B)
0.002
done
clear
C)
0.6
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 154) If the dissociation constant of two acids are \[2.7\times {{10}^{-4}}\]and\[3\times {{10}^{-5}}\]then the ratio of their relative strength will be
A)
\[9:1\]
done
clear
B)
\[3:1\]
done
clear
C)
\[3:2\]
done
clear
D)
\[9:2\]
done
clear
View Answer play_arrow
question_answer 155) Colour of\[ZnS{{O}_{4}}\]is white. It is due to
A)
completely filled\[{{d}^{10}}\]orbitals
done
clear
B)
magnetic moment\[\mu =0\]
done
clear
C)
coupling of spin and transfer of electron
done
clear
D)
pseudo inert configuration
done
clear
View Answer play_arrow
question_answer 156) Solution of\[C{{H}_{3}}COON{{H}_{4}}\]is
A)
amphoteric
done
clear
B)
neutral
done
clear
C)
basic
done
clear
D)
acidic
done
clear
View Answer play_arrow
question_answer 157) At 373K value of\[{{K}_{sp}}\]of\[AgCl\]is\[1.44\times {{10}^{-4}},\] then the solubility will be
A)
\[1.2\times {{10}^{-2}}\]
done
clear
B)
\[1.2\times {{10}^{-4}}\]
done
clear
C)
\[72\times {{10}^{-2}}\]
done
clear
D)
\[72\times {{10}^{-4}}\]
done
clear
View Answer play_arrow
question_answer 158) Alcohol does not completely neutralize with which of the following?
A)
Vitamin
done
clear
B)
Amyi alcohol
done
clear
C)
Castrol oil
done
clear
D)
Aniline
done
clear
View Answer play_arrow
question_answer 159) On heating\[N{{H}_{3}}\]with ethyl acetate, then the product obtained will be will obtain
A)
ethyl amine
done
clear
B)
ethanamide
done
clear
C)
ammonium acetate
done
clear
D)
acetic acid
done
clear
View Answer play_arrow
question_answer 160) For the polymerization of ethylene, the suitable condition is
A)
only high temperature
done
clear
B)
only catalyst
done
clear
C)
only high pressure
done
clear
D)
high temperature and high pressure
done
clear
View Answer play_arrow
question_answer 161) Keto-enol isomerism shows
A)
\[{{C}_{6}}{{H}_{5}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-{{C}_{6}}{{H}_{5}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{(C{{H}_{3}})}_{3}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-H\]
done
clear
View Answer play_arrow
question_answer 162) In the presence of KOH, phenol reacts with\[{{K}_{2}}{{S}_{2}}{{O}_{8}}\]to form the product
A)
benzene
done
clear
B)
1, 4-dihydroxy benzene
done
clear
C)
benzoic acid
done
clear
D)
4-hydroxy benzoic acid
done
clear
View Answer play_arrow
question_answer 163) Polyacetylene is used
A)
in the formation of medicine
done
clear
B)
in the formation of solid fuel
done
clear
C)
in the formation of insecticide
done
clear
D)
it is not possible
done
clear
View Answer play_arrow
question_answer 164) On the reaction of acetone and phenyl hydrazine the product will form
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}-N{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,=N-\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{N}}\,-{{C}_{6}}{{H}_{5}}\]
done
clear
C)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,=N-N{{H}_{2}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 165) On the reaction of butyric acid with\[{{H}_{2}}{{O}_{2}},\]the product will form
A)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
B)
\[\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{2}}-C{{H}_{2}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-OH\]
done
clear
C)
\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-OH\]
done
clear
D)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-OH\]
done
clear
View Answer play_arrow
question_answer 166) Acid in which\[COOH\]group is not present is
A)
aspirin
done
clear
B)
anthranilic acid
done
clear
C)
picric acid
done
clear
D)
amino acid
done
clear
View Answer play_arrow
question_answer 167) Sodium lauryl sulphate is
A)
cationic detergent
done
clear
B)
anionic detergent
done
clear
C)
foam active
done
clear
D)
neutral detergent
done
clear
View Answer play_arrow
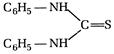
question_answer 168) \[2{{C}_{6}}{{H}_{5}}N{{H}_{2}}+C{{S}_{2}}+2KOH\xrightarrow[{}]{{}}\] Main product, the main product will be
A)
\[{{C}_{6}}{{H}_{5}}-N=C=S\]
done
clear
B)
done
clear
C)
\[{{C}_{6}}{{H}_{5}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-N{{H}_{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 169) In the reaction, \[6C{{H}_{2}}=C{{H}_{2}}+{{B}_{2}}{{H}_{6}}\xrightarrow[{}]{{}}2\left( H-\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{C}}}\,-\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{C}}}\, \right)-B\] the carb-boron product known as alkyl borane, on oxidation gives alcohols. This was made by which scientist?
A)
Brown and Benzamine
done
clear
B)
Jvifail
done
clear
C)
Methason
done
clear
D)
Suberao
done
clear
View Answer play_arrow
question_answer 170) In the presence of sunlight, when hot toluene reacts with excess of\[C{{l}_{2}},\]the product will be
A)
chloro benzene
done
clear
B)
benzal chloride
done
clear
C)
benzyl chloride
done
clear
D)
benzo trichloride
done
clear
View Answer play_arrow
question_answer 171) Monomer of natural rubber is
A)
\[C{{H}_{2}}=C=C{{H}_{2}}\]
done
clear
B)
\[C{{H}_{2}}=CH-Cl\]
done
clear
C)
\[C{{H}_{2}}=\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,-CH=C{{H}_{2}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 172) Monomers of nylon-6 6 are
A)
isoprene + chloroprene
done
clear
B)
adipic acid + ethylene glycol
done
clear
C)
adipic acid + hexamethylene diamine
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 173) Nylon-6 is formed from caprolactum. Formula of caprolactum is
A)
done
clear
B)
done
clear
C)
done
clear
D)
\[COOH{{(C{{H}_{2}})}_{6}}COOH\]
done
clear
View Answer play_arrow
question_answer 174) In the esterification of alcohol, the order of reactivity is
A)
\[2{}^\circ >3{}^\circ >1{}^\circ \]
done
clear
B)
\[3{}^\circ >2{}^\circ >1{}^\circ \]
done
clear
C)
\[1{}^\circ >2{}^\circ >3{}^\circ \]
done
clear
D)
\[1{}^\circ >2{}^\circ >3{}^\circ \]
done
clear
View Answer play_arrow
question_answer 175) 1, 3-pentadiene is more stable than 1, 4-pentadiene because
A)
it is a conjugated diene
done
clear
B)
its dipole moment is more
done
clear
C)
Both are functional and position isomers
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 176) On complete oxidation ether gives
A)
\[{{C}_{2}}H.pH\]
done
clear
B)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
C)
\[C{{O}_{2}}+{{H}_{2}}O\]
done
clear
D)
\[C{{H}_{3}}COOH\]
done
clear
View Answer play_arrow
question_answer 177) In the electrolysis of sodium acetate,\[{{H}_{2}}\]gas releases at cathode and\[{{C}_{2}}{{H}_{6}}\]gas at anode. This reaction is known as
A)
Frenkland
done
clear
B)
Kolbe
done
clear
C)
Clemmensen
done
clear
D)
Wolff-Kishner
done
clear
View Answer play_arrow
question_answer 178) Reactivity order of HX with alcohol is
A)
\[HCl>HBr>HI>HF\]
done
clear
B)
\[HF>HCl>HBr>HI\]
done
clear
C)
\[HI>HCl>HBr>HF\]
done
clear
D)
\[HI>HBr>HCl>HF\]
done
clear
View Answer play_arrow
question_answer 179) Anhydrous formic acid cannot be obtained from fractional distillation of aqueous HCOOH because
A)
it forms complex with water
done
clear
B)
its boiling point is 373.4 K
done
clear
C)
it is soluble in water
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 180) TEL added in petrol and other fuels because
A)
it reduces heat
done
clear
B)
it reduces coolness
done
clear
C)
it reduces pressure
done
clear
D)
it reduces knocking property
done
clear
View Answer play_arrow
question_answer 181) Which of the following shows optical isomerism?
A)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\overset{\begin{smallmatrix} Br \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\overset{\begin{smallmatrix} Br \\ | \end{smallmatrix}}{\mathop{C}}}\,-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-OH\]
done
clear
C)
\[H-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\overset{\begin{smallmatrix} Br \\ | \end{smallmatrix}}{\mathop{C}}}\,-H\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-OH\]
done
clear
View Answer play_arrow
question_answer 182) Which of the following reagent used in the formation of ethane amine from propanamide?
A)
\[B{{r}_{2}}+KOH\]
done
clear
B)
\[Na+{{C}_{2}}{{H}_{5}}OH\]
done
clear
C)
\[LiAl{{H}_{4}}\]
done
clear
D)
\[AlC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 183) In the reaction. \[HCOOH+C{{H}_{3}}COOH\xrightarrow[\Delta ]{MnO}X;\] Product X is
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-H\]
done
clear
C)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-O-{{C}_{2}}{{H}_{5}}\]
done
clear
D)
\[H-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-O-{{C}_{2}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 184) \[{{C}_{6}}{{H}_{5}}OH\]and\[{{C}_{6}}{{H}_{5}}COOH\]can be distinguished by
A)
\[FeC{{l}_{3}}\]
done
clear
B)
Na metal
done
clear
C)
\[NaOH\]
done
clear
D)
litmus
done
clear
View Answer play_arrow
question_answer 185) For increase in strong basic nature of aniline, which group should attach on para position?
A)
\[-OC{{H}_{3}}\]
done
clear
B)
\[-C{{H}_{3}}\]
done
clear
C)
\[-N{{O}_{2}}\]
done
clear
D)
\[-Cl\]
done
clear
View Answer play_arrow
question_answer 186) Which of the following alkene gives acetone on ozonolysis?
A)
2-methyl propene
done
clear
B)
2-methyl-l-butene
done
clear
C)
2-butene
done
clear
D)
Ethene
done
clear
View Answer play_arrow
question_answer 187) Which of the following cannot be obtained by\[CHC{{l}_{3}}\]?
A)
Freon
done
clear
B)
Chloropicrin
done
clear
C)
Chloretone
done
clear
D)
Salicyldehyde
done
clear
View Answer play_arrow
question_answer 188) Which of the following doesn't show Friedel-Craft reaction?
A)
Nitrobenzene
done
clear
B)
Toluene
done
clear
C)
Benzene
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 189) Increasing reactivity order of nucleophilic addition reaction of \[H-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-H;C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-H\] and\[C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\]will be
A)
\[HCHO>C{{H}_{3}}CHO>C{{H}_{3}}COC{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}CHO>HCHO>C{{H}_{3}}COC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}>C{{H}_{3}}CHO>HCHO\]
done
clear
D)
\[C{{H}_{3}}CHO>C{{H}_{3}}COC{{H}_{3}}>HCHO\]
done
clear
View Answer play_arrow
question_answer 190) Purity of organic compound is determined by
A)
molecular weight
done
clear
B)
boiling point
done
clear
C)
density
done
clear
D)
solubility in water
done
clear
View Answer play_arrow
question_answer 191) Which of the following reaction is not stereospecific?
A)
Electrophilic substitution
done
clear
B)
\[{{S}_{N}}1\]
done
clear
C)
\[{{S}_{N}}2\]
done
clear
D)
Addition of\[B{{r}_{2}}\]on ethylene
done
clear
View Answer play_arrow
question_answer 192) The product will be obtained from the reaction of\[{{C}_{2}}{{H}_{2}}\]with\[AsC{{l}_{3}}\]in the presence of\[(AlC{{l}_{3}}+HCl)\]
A)
Lewisite
done
clear
B)
Electrophile
done
clear
C)
Insecticide
done
clear
D)
Nucleophite
done
clear
View Answer play_arrow
question_answer 193) \[R-C\equiv N\xrightarrow[{}]{Sn+HCl}X\xrightarrow[{}]{{{H}_{2}}O}Y\]reaction is known as
A)
Rosenmund's reaction
done
clear
B)
Stephen's reaction
done
clear
C)
Clemmensen reduction
done
clear
D)
Cannizaro reaction
done
clear
View Answer play_arrow
question_answer 194) \[C{{H}_{3}}-Mg-X+H-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-H\xrightarrow[{}]{{}}X\xrightarrow[{}]{{{H}_{2}}O}Y\] Product is
A)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}COOH\]
done
clear
D)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-O-{{C}_{2}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 195) \[2C{{H}_{3}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-H\xrightarrow[{}]{Dil.base}X\xrightarrow[{}]{\Delta }Y\] Product ?Y? is
A)
\[C{{H}_{3}}-CH=CH-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-H\]
done
clear
B)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-H\]
done
clear
C)
\[C{{H}_{3}}-CH=CH-COOH\]
done
clear
D)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}-COOH\]
done
clear
View Answer play_arrow
question_answer 196) \[2Cr{{(OH)}_{3}}+4O{{H}^{-}}+KI{{O}_{3}}\to 2CrO_{4}^{2-}\]\[+5{{H}_{2}}O+KI\] Equivalent weight of\[KI{{O}_{3}}\]in the above reaction is
A)
molecular weight
done
clear
B)
\[\frac{molecular\text{ }weight}{3}\]
done
clear
C)
\[\frac{molecular\text{ }weight}{6}\]
done
clear
D)
\[\frac{molecular\text{ }weight}{2}\]
done
clear
View Answer play_arrow
question_answer 197) Which metal is present in chlorophyll?
A)
Cr
done
clear
B)
Co
done
clear
C)
Mg
done
clear
D)
Fe
done
clear
View Answer play_arrow
question_answer 198) Which gas is used for the cutting and welding of metal?
A)
Acetylene
done
clear
B)
Methane
done
clear
C)
Carbon dioxide
done
clear
D)
Neon
done
clear
View Answer play_arrow
question_answer 199) Which of the following will give silver mirror test?
A)
Glucose
done
clear
B)
Fructose
done
clear
C)
Starch
done
clear
D)
Sucrose
done
clear
View Answer play_arrow
question_answer 200) Conjugated acid of\[NH_{2}^{-}\]is
A)
\[NH_{4}^{+}\]
done
clear
B)
\[N{{H}_{4}}OH\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[{{N}_{2}}{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 201) \[\underset{x\to 0}{\mathop{\lim }}\,\frac{{{e}^{\tan x}}-{{e}^{x}}}{\tan x-x}\]is equal to
A)
0
done
clear
B)
1
done
clear
C)
\[e\]
done
clear
D)
\[1/e\]
done
clear
View Answer play_arrow
question_answer 202) \[\underset{x\to 0}{\mathop{\lim }}\,\frac{\sqrt{\frac{1-\cos 2x}{2}}}{x}\]is equal to
A)
1
done
clear
B)
\[-1\]
done
clear
C)
0
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 203) \[\underset{x\to 0}{\mathop{\lim }}\,\frac{x}{{{\tan }^{-1}}2x}\]is equal to
A)
2
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
0
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 204) If\[y=a{{x}^{n+1}}+b{{x}^{-n}},\]then\[\frac{{{x}^{2}}{{d}^{2}}y}{d{{x}^{2}}}\]is equal to
A)
\[ny\]
done
clear
B)
\[{{n}^{2}}y\]
done
clear
C)
\[n(n-1)y\]
done
clear
D)
\[n(n+1)y\]
done
clear
View Answer play_arrow
question_answer 205) \[\frac{d}{dx}\left[ \log \left\{ {{e}^{x}}{{\left( \frac{x-2}{x+2} \right)}^{3/4}} \right\} \right]\]is equal to
A)
1
done
clear
B)
\[\frac{{{x}^{2}}+1}{{{x}^{2}}-4}\]
done
clear
C)
\[\frac{{{x}^{2}}-1}{{{x}^{2}}-4}\]
done
clear
D)
\[\frac{{{x}^{2}}-1}{{{x}^{2}}-4}{{e}^{x}}\]
done
clear
View Answer play_arrow
question_answer 206) \[\int{\frac{1+{{\tan }^{2}}x}{1-{{\tan }^{2}}x}}dx\]is equal to
A)
\[\log \left( \frac{1-\tan x}{1+\tan x} \right)+c\]
done
clear
B)
\[\log \left( \frac{1+\tan x}{1-\tan x} \right)+c\]
done
clear
C)
\[\frac{1}{2}\log \left( \frac{1-\tan x}{1+\tan x} \right)+c\]
done
clear
D)
\[\frac{1}{2}\log \left( \frac{1+\tan x}{1-\tan x} \right)+c\]
done
clear
View Answer play_arrow
question_answer 207) If\[{{a}^{2}}{{x}^{4}}+{{b}^{2}}{{y}^{4}}={{c}^{6}},\]then maximum value of\[xy\] is
A)
\[\frac{{{c}^{3}}}{2ab}\]
done
clear
B)
\[\frac{{{c}^{3}}}{\sqrt{2ab}}\]
done
clear
C)
\[\frac{{{c}^{3}}}{ab}\]
done
clear
D)
\[\frac{{{c}^{3}}}{\sqrt{ab}}\]
done
clear
View Answer play_arrow
question_answer 208) Maximum value of\[px+qy,\]when\[xy={{r}^{2}},\]is
A)
\[2r\sqrt{pq}\]
done
clear
B)
\[2pq\sqrt{r}\]
done
clear
C)
\[-2r\sqrt{pq}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 209) If\[f(x)={{x}^{2}}+1,\] then the value of\[{{f}^{-1}}(17)\]and\[{{f}^{-1}}(-3)\]are respectively
A)
4, 1
done
clear
B)
4, 0
done
clear
C)
3, 2
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 210) If\[a=\cos \alpha +i\sin \alpha ,\]\[b=\cos \beta +i\sin \beta ,\]\[c=\cos \gamma +i\sin \gamma \]and\[\frac{b}{c}+\frac{c}{a}+\frac{a}{b}=1\]then \[\cos (\beta -\gamma )+\cos (\gamma -\alpha )+\cos (\alpha -\beta )\]is equal to
A)
\[\frac{3}{2}\]
done
clear
B)
\[-\frac{3}{2}\]
done
clear
C)
\[0\]
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 211) \[{{\left[ \frac{1+\cos \frac{\pi }{8}+i\sin \frac{\pi }{8}}{1+\cos \frac{\pi }{8}-i\sin \frac{\pi }{8}} \right]}^{8}}\]is equal to
A)
0
done
clear
B)
1
done
clear
C)
\[-1\]
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 212) \[{{i}^{n}}+{{i}^{n+1}}+{{i}^{n+2}}+{{i}^{n+3}},(n\in N)\]is equal to
A)
4
done
clear
B)
1
done
clear
C)
0
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 213) If\[2(y-a)\]is the harmonic mean of\[y-x\]and \[y-z,\]then\[x-a,\text{ }y-a,\text{ }z-a\]are in
A)
AP
done
clear
B)
GP
done
clear
C)
HP
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 214) If\[{{a}^{x}}={{b}^{y}}={{c}^{z}}={{d}^{u}}\]and a, b, c, d are in GP, then\[x,\text{ }y,\text{ }z\]are in
A)
AP
done
clear
B)
GP
done
clear
C)
HP
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 215) \[lo{{g}_{3}}2,\text{ }lo{{g}_{6}}2,\text{ }lo{{g}_{12}}2\]are in
A)
AP
done
clear
B)
GP
done
clear
C)
HP
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 216) Angle between the planes\[2x-y+z=6\]and \[x+y+2z=3\]is
A)
\[\frac{\pi }{3}\]
done
clear
B)
\[\frac{\pi }{6}\]
done
clear
C)
\[\frac{\pi }{2}\]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 217) \[\int_{0}^{\pi /2}{\frac{\sqrt[3]{{{\sin }^{2}}x}dx}{\sqrt[3]{{{\sin }^{2}}x}\sqrt[3]{{{\cos }^{2}}x}}}\]is equal to
A)
\[\frac{\pi }{4}\]
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
\[\pi \]
done
clear
D)
\[2\pi \]
done
clear
View Answer play_arrow
question_answer 218) \[\int_{-1}^{1}{|1-x|}\,dx\]is equal to
A)
0
done
clear
B)
\[-2\]
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 219) \[\int_{0}^{1}{\frac{{{\tan }^{-1}}xdx}{1+{{x}^{2}}}}\]is equal to
A)
\[\frac{\pi }{4}\]
done
clear
B)
\[\frac{\pi }{8}\]
done
clear
C)
\[\frac{{{\pi }^{2}}}{16}\]
done
clear
D)
\[\frac{{{\pi }^{2}}}{32}\]
done
clear
View Answer play_arrow
question_answer 220) Area of the region bounded by the curve \[y=x,\text{ }x-\]axis and abscissas\[x=-1\]and\[x=2\]is
A)
0 sq unit
done
clear
B)
3/2 sq unit
done
clear
C)
5/2 sq units
done
clear
D)
- 5/2 sq unit
done
clear
View Answer play_arrow
question_answer 221) The value of \[\underset{x\to 1}{\mathop{\lim }}\,(1-x)\tan \frac{\pi x}{2}\]is equal to
A)
\[1\]
done
clear
B)
\[\infty \]
done
clear
C)
\[\frac{2}{\pi }\]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 222) If\[z=i\log (2-\sqrt{3}),\]then\[cos\text{ }z\]is equal to
A)
0
done
clear
B)
2
done
clear
C)
4
done
clear
D)
\[-2\]
done
clear
View Answer play_arrow
question_answer 223) Tangent and normal at any point P of the parabola meet the axes at T and G respectively, then
A)
\[ST=SG.SP\]
done
clear
B)
\[ST\ne SG=SP\]
done
clear
C)
\[ST=SG\ne SP\]
done
clear
D)
\[ST=SG=SP\]
done
clear
View Answer play_arrow
question_answer 224) Domain of the function\[f(x)=\log (\sqrt{x-4}+\]\[\sqrt{6-x})\]is
A)
\[[4,\infty ]\]
done
clear
B)
\[(-\infty ,6)\]
done
clear
C)
[4, 6]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 225) Inverse of the function\[\frac{{{10}^{x}}-{{10}^{-x}}}{{{10}^{x}}+{{10}^{-x}}}\]is
A)
\[{{\log }_{10}}(2-x)\]
done
clear
B)
\[\frac{1}{2}{{\log }_{10}}\left( \frac{1+x}{1-x} \right)\]
done
clear
C)
\[\frac{1}{2}{{\log }_{10}}(2x-1)\]
done
clear
D)
\[\frac{1}{4}{{\log }_{10}}\left( \frac{2x}{2-x} \right)\]
done
clear
View Answer play_arrow
question_answer 226) Domain of the function \[f(x)={{\log }_{3+x}}({{x}^{2}}-1)\]is
A)
\[(-3,-1)\cup (1,\infty )\]
done
clear
B)
\[(-3,-1)\cup [1,\infty )\]
done
clear
C)
\[(-3,-2)\cup (-2,-1)\cup (1,\infty )\]
done
clear
D)
\[[-3,-2)\cup (-2,-1)\cup [1,\infty )\]
done
clear
View Answer play_arrow
question_answer 227) If\[n=3k\]and\[1,\omega ,{{\omega }^{2}}\]are the cube roots of unity, then the value of the determinant \[\left| \begin{matrix} 1 & {{\omega }^{n}} & {{\omega }^{2n}} \\ {{\omega }^{2n}} & 1 & {{\omega }^{n}} \\ {{\omega }^{n}} & {{\omega }^{2n}} & 1 \\ \end{matrix} \right|\]is
A)
0
done
clear
B)
\[\omega \]
done
clear
C)
\[{{\omega }^{2}}\]
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 228) One root of the equation \[\left| \begin{matrix} 3-x & -6 & 3 \\ -6 & 3-x & 3 \\ 3 & 3 & -6-x \\ \end{matrix} \right|=0\]is
A)
6
done
clear
B)
3
done
clear
C)
0
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 229) Three coins are tossed together, then the probability of getting atleast one tail is
A)
\[\frac{1}{8}\]
done
clear
B)
\[\frac{7}{8}\]
done
clear
C)
\[\frac{1}{2}\]
done
clear
D)
\[\frac{3}{4}\]
done
clear
View Answer play_arrow
question_answer 230) A card is drawn randomly from a pack of 52 cards, then the probability that the card is king or spade is
A)
\[\frac{4}{13}\]
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
\[\frac{1}{4}\]
done
clear
D)
\[\frac{3}{4}\]
done
clear
View Answer play_arrow
question_answer 231) Angle between those two lines, in which direction comes are represented by \[l+m+n=0\]and\[{{l}^{2}}+{{m}^{2}}-{{n}^{2}}=0\]is
A)
\[\frac{2\pi }{3}\]
done
clear
B)
\[\frac{\pi }{6}\]
done
clear
C)
\[\frac{5\pi }{6}\]
done
clear
D)
\[\frac{\pi }{3}\]
done
clear
View Answer play_arrow
question_answer 232) If \[P(A)=0.25,P(B)=0.50\]and \[P(B/A)=0.6,\]then the value of P(A/B) is
A)
0.3
done
clear
B)
0.35
done
clear
C)
0.15
done
clear
D)
0.5
done
clear
View Answer play_arrow
question_answer 233) If \[f(x)=|x{{|}^{3}},\]then\[f'(0)\]is equal to
A)
0
done
clear
B)
1
done
clear
C)
\[f'(1)\]
done
clear
D)
\[f'\,'(1)\]
done
clear
View Answer play_arrow
question_answer 234) \[{{\cosh }^{-1}}x\]is equal to
A)
\[\log (x+\sqrt{{{x}^{2}}+1})\]
done
clear
B)
\[\log (x+\sqrt{{{x}^{2}}-1})\]
done
clear
C)
\[\log (x+\sqrt{1-{{x}^{2}}})\]
done
clear
D)
\[\log (x-\sqrt{{{x}^{2}}-1})\]
done
clear
View Answer play_arrow
question_answer 235) There are 16 points in a plane, out of which 6 points are collinear. Then, how many straight lines can be drawn using these lines.
A)
106
done
clear
B)
105
done
clear
C)
60
done
clear
D)
55
done
clear
View Answer play_arrow
question_answer 236) \[{{(1+i)}^{8}}+{{(1-i)}^{8}}\]is equal to
A)
16
done
clear
B)
\[-16\]
done
clear
C)
32
done
clear
D)
\[-32\]
done
clear
View Answer play_arrow
question_answer 237) \[\int{\frac{{{e}^{m{{\tan }^{-1}}x}}}{1+{{x}^{2}}}}dx\]is equal to
A)
\[{{e}^{m+{{\tan }^{-1}}x}}\]
done
clear
B)
\[{{e}^{{{\tan }^{-1}}x}}\]
done
clear
C)
\[\frac{1}{m}{{\tan }^{-1}}x\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 238) The adjoint matrix of the matrix\[\left[ \begin{matrix} 1 & 8 \\ 2 & -5 \\ \end{matrix} \right]\]is given by
A)
\[\left[ \begin{matrix} -5 & -8 \\ -2 & 1 \\ \end{matrix} \right]\]
done
clear
B)
\[\left[ \begin{matrix} -5 & -2 \\ -8 & 1 \\ \end{matrix} \right]\]
done
clear
C)
\[\left[ \begin{matrix} 5 & -8 \\ -2 & 1 \\ \end{matrix} \right]\]
done
clear
D)
\[\left[ \begin{matrix} 5 & -8 \\ -2 & -1 \\ \end{matrix} \right]\]
done
clear
View Answer play_arrow
question_answer 239) If p and q are the roots of the equation \[{{x}^{2}}+px+q=0,\]then
A)
\[p=1\]
done
clear
B)
\[p=-2\]
done
clear
C)
\[p=0\]and 1
done
clear
D)
\[p=-2\]and 0
done
clear
View Answer play_arrow
question_answer 240) Coefficient of\[{{x}^{4}}\]in the expansion of \[{{(1+x+{{x}^{2}}+{{x}^{3}})}^{n}}\]is
A)
\[^{n}{{C}_{4}}\]
done
clear
B)
\[^{n}{{C}_{4}}{{+}^{n}}{{C}_{2}}\]
done
clear
C)
\[^{n}{{C}_{4}}{{+}^{n}}{{C}_{2}}{{+}^{n}}{{C}_{4}}^{n}{{C}_{2}}\]
done
clear
D)
\[^{n}{{C}_{4}}{{+}^{n}}{{C}_{2}}{{+}^{n}}{{C}_{1}}^{n}{{C}_{2}}\]
done
clear
View Answer play_arrow
question_answer 241) \[\int_{0}^{\pi /2}{\log \tan xdx}\]is equal to
A)
\[\frac{\pi }{4}\]
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
0
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 242) Focus and directrix of the parabola\[{{x}^{2}}=-8ay\]are
A)
\[(-2a,0)\]and\[x=4\]
done
clear
B)
\[(0,2a)\]and\[y=-2a\]
done
clear
C)
\[(0,-2a)\]and\[y=2a\]
done
clear
D)
\[[2a,0)\]and\[x=-2a\]
done
clear
View Answer play_arrow
question_answer 243) Coefficient of\[{{x}^{4}}\]in the expansion of \[{{\left( \frac{x}{2}-\frac{3}{{{x}^{2}}} \right)}^{10}}\]is
A)
\[\frac{504}{259}\]
done
clear
B)
\[\frac{450}{263}\]
done
clear
C)
\[\frac{405}{256}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 244) Coordinates of that point on\[x-\]axis which is at a perpendicular distance 'a' from the line \[\frac{x}{a}+\frac{y}{b}=1,\]are
A)
\[(\pm a,0)\]
done
clear
B)
\[\left( a\pm \frac{a}{b}\sqrt{{{a}^{2}}+{{b}^{2}}},0 \right)\]
done
clear
C)
\[\left( a+\frac{a}{b}\sqrt{{{a}^{2}}+{{b}^{2}}},0 \right)\]
done
clear
D)
\[\left( a-\frac{a}{b}\sqrt{{{a}^{2}}+{{b}^{2}}},0 \right)\]
done
clear
View Answer play_arrow
question_answer 245) Equation of tangent to the circle\[{{x}^{2}}+{{y}^{2}}={{a}^{2}}\] which is parallel to the line\[y=mx+c,\]is
A)
\[my=x\pm a\sqrt{1+{{m}^{2}}}\]
done
clear
B)
\[y=mx\pm \sqrt{1+{{m}^{2}}}\]
done
clear
C)
\[y=mx\pm a\sqrt{1+{{m}^{2}}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 246) Which one of the following is correct?
A)
\[cos\text{ }ix=i\text{ }cosh\text{ }x\]
done
clear
B)
\[\tan ix=tanh\text{ }x\]
done
clear
C)
\[\sin \text{ }ix=i\text{ }\sin \text{ }x\]
done
clear
D)
\[cos\text{ }ix=cosh\text{ }x\]
done
clear
View Answer play_arrow
question_answer 247) Equation of line perpendicular to\[x=c\]is
A)
\[y=0\]
done
clear
B)
\[x=0\]
done
clear
C)
\[x=y\]
done
clear
D)
\[x=d\]
done
clear
View Answer play_arrow
question_answer 248) \[{{(\sin \theta +i\cos \theta )}^{n}}\] is equal to
A)
\[{{i}^{n}}(i\sin \theta +\cos \theta )\]
done
clear
B)
\[(\sin n\theta +i\cos n\theta )\]
done
clear
C)
\[\cos n\left( \frac{\pi }{2}-\theta \right)+i\sin n\left( \frac{\pi }{2}-\theta \right)\]
done
clear
D)
\[\cos \theta +i\sin n\theta \]
done
clear
View Answer play_arrow
question_answer 249) If A is a square matrix, then\[A+{{A}^{T}}\]is
A)
symmetric matrix
done
clear
B)
anti-symmetric matrix
done
clear
C)
diagonal matrix
done
clear
D)
triangular matrix
done
clear
View Answer play_arrow
question_answer 250) If a, b are two constant positive integers such that\[f(a+x)=b+[{{b}^{3}}+1-3{{b}^{2}}f(x)+3b{{\{f(x)\}}^{2}}\]\[-\{f{{(x)}^{3}}\}{{]}^{1/3}},\]for every\[x\]. Then,\[f(x)\]is a periodic function with the period
A)
\[a\]
done
clear
B)
\[2a\]
done
clear
C)
\[b\]
done
clear
D)
\[2b\]
done
clear
View Answer play_arrow
question_answer 251) If\[\overrightarrow{A}.\overrightarrow{B}=\overrightarrow{A}.\overrightarrow{C},\]then difference of\[\overrightarrow{B}\]and\[\overrightarrow{C}\]and\[\overrightarrow{A}\]are
A)
parallel
done
clear
B)
perpendicular
done
clear
C)
coplanar
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 252) If\[A=\left[ \begin{matrix} \cos \alpha & \sin \alpha \\ -\sin \alpha & \cos \alpha \\ \end{matrix} \right],\]then\[{{A}^{2}}\]is equal to
A)
\[\left[ \begin{matrix} \cos 2\alpha & \sin 2\alpha \\ -\sin 2\alpha & \cos 2\alpha \\ \end{matrix} \right]\]
done
clear
B)
\[\left[ \begin{matrix} {{\cos }^{2}}\alpha & -{{\sin }^{2}}\alpha \\ -{{\sin }^{2}}\alpha & {{\cos }^{2}}\alpha \\ \end{matrix} \right]\]
done
clear
C)
\[\left[ \begin{matrix} {{\sin }^{2}}\alpha & {{\cos }^{2}}\alpha \\ -{{\cos }^{2}}\alpha & {{\sin }^{2}}\alpha \\ \end{matrix} \right]\]
done
clear
D)
\[\left[ \begin{matrix} 1 & 0 \\ 0 & 1 \\ \end{matrix} \right]\]
done
clear
View Answer play_arrow
question_answer 253) Argument of\[\frac{1+\sqrt{3}i}{\sqrt{3}+i}\]is
A)
\[\frac{\pi }{3}\]
done
clear
B)
\[\frac{\pi }{2}\]
done
clear
C)
\[0\]
done
clear
D)
\[\frac{\pi }{6}\]
done
clear
View Answer play_arrow
question_answer 254) The tangential point, where the line\[y=mx+c\]touches the parabola\[{{y}^{2}}=4ax,\]is
A)
\[\left( \frac{a}{{{m}^{2}}},\frac{-2a}{m} \right)\]
done
clear
B)
\[\left( -\frac{a}{{{m}^{2}}},-\frac{2a}{m} \right)\]
done
clear
C)
\[\left( -\frac{a}{{{m}^{2}}},-\frac{2a}{m} \right)\]
done
clear
D)
\[\left( \frac{a}{{{m}^{2}}},\frac{2a}{m} \right)\]
done
clear
View Answer play_arrow
question_answer 255) \[\frac{(\cos \alpha +i\sin \alpha )(\cos \beta +i\sin \beta )}{(\cos \gamma +i\sin \gamma )(\cos \delta +i\sin \delta )}\]is equal to
A)
\[\cos (\alpha +\beta -\gamma -\delta )-i\sin (\alpha +\beta -\gamma -\delta )\]
done
clear
B)
\[\cos (\alpha +\beta -\gamma +\delta )+i\sin (\alpha +\beta -\gamma +\delta )\]
done
clear
C)
\[\sin (\alpha +\beta -\gamma -\delta )+i\cos (\alpha +\beta -\gamma -\delta )\]
done
clear
D)
\[\cos (\alpha +\beta -\gamma -\delta )+i\sin (\alpha +\beta -\gamma -\delta )\]
done
clear
View Answer play_arrow
question_answer 256) \[sinh\text{ }3x\] is equal to
A)
\[4sin{{h}^{3}}x-3sinh\text{ }x\]
done
clear
B)
\[3\text{ }sin{{h}^{3}}x+4\text{ }sinh\text{ }x\]
done
clear
C)
\[3\text{ }sinh\text{ }x+4\text{ }sin{{h}^{3}}x\]
done
clear
D)
\[3\text{ }sin{{h}^{3}}x-4\text{ }sinh\text{ }x\]
done
clear
View Answer play_arrow
question_answer 257) If\[z=\frac{\sqrt{3}+i}{-2},\]then\[{{z}^{69}}\]is equal to
A)
1
done
clear
B)
2
done
clear
C)
\[-i\]
done
clear
D)
\[-1\]
done
clear
View Answer play_arrow
question_answer 258) If\[x+\frac{1}{x}=2\cos \theta ,\]then\[x\]is equal to
A)
\[cos\text{ }\theta +i\text{ }sin\text{ }\theta \]
done
clear
B)
\[cos\text{ }\theta -i\text{ }sin\text{ }\theta \]
done
clear
C)
\[cos\text{ }\theta \pm i\text{ }sin\text{ }\theta \]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 259) Projection of a line on the axes are 3, 4, 5 respectively, then its length is
A)
12
done
clear
B)
50
done
clear
C)
\[5\sqrt{2}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 260) If\[\overrightarrow{a}\]and\[\overrightarrow{b}\]are the adjacent sides of a rhombus, then
A)
\[\overrightarrow{a}.\overrightarrow{a}=\overrightarrow{b}.\overrightarrow{b}\]
done
clear
B)
\[\overrightarrow{a}.\overrightarrow{b}=0\]
done
clear
C)
\[\overrightarrow{a}\times \overrightarrow{b}=0\]
done
clear
D)
\[\overrightarrow{a}.\overrightarrow{a}=0\]
done
clear
View Answer play_arrow
question_answer 261) \[\overrightarrow{a}[\overrightarrow{a}\times (\overrightarrow{a}\times \overrightarrow{b})]\]is equal to
A)
\[(\overrightarrow{a}.\overrightarrow{a})(\overrightarrow{a}\times \overrightarrow{b})\]
done
clear
B)
\[(\overrightarrow{a}.\overrightarrow{a})(\overrightarrow{b}\times \overrightarrow{a})\]
done
clear
C)
\[(\overrightarrow{b}.\overrightarrow{b})(\overrightarrow{a}\times \overrightarrow{b})\]
done
clear
D)
\[(\overrightarrow{b}.\overrightarrow{b})(\overrightarrow{b}\times \overrightarrow{a})\]
done
clear
View Answer play_arrow
question_answer 262) \[\overrightarrow{A}.(\overrightarrow{B}\times \overrightarrow{C})\]is equal to
A)
\[\overrightarrow{B}.(\overrightarrow{A}\times \overrightarrow{C})\]
done
clear
B)
\[\overrightarrow{B}.(\overrightarrow{C}\times \overrightarrow{A})\]
done
clear
C)
\[\overrightarrow{C}.(\overrightarrow{B}\times \overrightarrow{A})\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 263) If\[O\]is the origin and\[{{P}_{3}}\]is the mid point of\[{{P}_{1}}(2,-1)\]and\[{{P}_{2}}(-4,3),\]then\[{{\overset{\to }{\mathop{OP}}\,}_{3}}\]is equal to
A)
\[(-1,1)\]
done
clear
B)
\[(1,1)\]
done
clear
C)
\[(1,-1)\]
done
clear
D)
\[(-1,-1)\]
done
clear
View Answer play_arrow
question_answer 264) \[\frac{\cos \sqrt{x}}{\sqrt{x}}\]is equal to
A)
\[\sin \sqrt{x}+c\]
done
clear
B)
\[2\sin \sqrt{x}+c\]
done
clear
C)
\[-\sin \sqrt{x}+c\]
done
clear
D)
\[-2\sin \sqrt{x}+c\]
done
clear
View Answer play_arrow
question_answer 265) Sum of the infinite terms of the series\[1+\frac{4}{5}+\frac{7}{{{5}^{2}}}+\frac{10}{{{5}^{3}}}+....\]is
A)
\[\frac{16}{35}\]
done
clear
B)
\[\frac{11}{8}\]
done
clear
C)
\[\frac{35}{16}\]
done
clear
D)
\[\frac{8}{16}\]
done
clear
View Answer play_arrow
question_answer 266) Radical axis of the circles \[3{{x}^{2}}+3{{y}^{2}}-7x+8y+11=0\]and \[{{x}^{2}}+{{y}^{2}}-3x-4y+5=0\]is
A)
\[x+10y=2\]
done
clear
B)
\[x+10y+2=0\]
done
clear
C)
\[x+10y=8\]
done
clear
D)
\[x+10y+8=0\]
done
clear
View Answer play_arrow
question_answer 267) \[\underset{x\to 0}{\mathop{\lim }}\,\frac{x({{5}^{x}}-1)}{1-\cos x}\]is equal to
A)
\[4\text{ }log\text{ }5\]
done
clear
B)
\[2\text{ }log\text{ }5\]
done
clear
C)
\[\frac{1}{2}log\text{ }5\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 268) If the line\[x+2y=1\]is the polar of the circle \[{{x}^{2}}+{{y}^{2}}=5,\]then pole is
A)
\[(5,10)\]
done
clear
B)
\[(5,5)\]
done
clear
C)
\[(-5,10)\]
done
clear
D)
\[(-5,-10)\]
done
clear
View Answer play_arrow
question_answer 269) \[\cos (\alpha +i\beta )\]is equal to
A)
\[cos\text{ }\alpha \text{ }coch\text{ }\beta -2\text{ }sin\text{ }\alpha \text{ }sinh\text{ }\beta \]
done
clear
B)
\[cos\text{ }\alpha \text{ }cosh\text{ }\beta +2\text{ }sin\text{ }\alpha \text{ }sinh\text{ }\beta \]
done
clear
C)
\[cosh\text{ }\alpha \text{ }cos\text{ }\beta -2\text{ }sinh\text{ }\alpha \text{ }sin\text{ }\beta \]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 270) If\[\overrightarrow{A}=2\hat{i}-2\hat{j}-\hat{k}\]and\[\overrightarrow{B}=-\hat{i}+3\hat{j}+2\hat{k},\]then \[A\times B\]is equal to
A)
\[\hat{i}+2\hat{j}-4\hat{k}\]
done
clear
B)
\[-\hat{i}-3\hat{j}+4\hat{k}\]
done
clear
C)
\[-\hat{i}-5\hat{j}+4\hat{k}\]
done
clear
D)
\[-2\hat{i}-\hat{j}+\hat{k}\]
done
clear
View Answer play_arrow
question_answer 271) If\[\overrightarrow{A}=4\hat{i}+8\hat{j}-\hat{k},\overrightarrow{B}=3\hat{i}+2\hat{j}-\hat{k}\]and\[\overrightarrow{C}=5\hat{i}+4\hat{j}+\hat{k},\]then\[\overrightarrow{A}.(\overrightarrow{B}.\overrightarrow{C})\]is equal to
A)
\[88\hat{i}+176\hat{j}-22\hat{k}\]
done
clear
B)
\[22\hat{i}+44\hat{j}-11\hat{k}\]
done
clear
C)
\[\hat{i}+4\hat{j}-\hat{k}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 272) If A and B are two matrices such that\[AB=0,\]then
A)
\[A\ne O\]or\[B=O\]
done
clear
B)
\[A=O\]and\[B=O\]
done
clear
C)
it is not necessary that\[A=O\]or\[B=O\]
done
clear
D)
all the statements are incorrect
done
clear
View Answer play_arrow
question_answer 273) If\[\overrightarrow{a}\]and\[\overrightarrow{b}\]are non-collinear vectors and \[x\overrightarrow{a}+y\overrightarrow{b}=0,\]then
A)
\[x=0\]but it is not necessary for y to be 0
done
clear
B)
not dependent on\[x\]and \[y\]
done
clear
C)
\[x=0,y=0\]
done
clear
D)
dependent on\[x\]and y
done
clear
View Answer play_arrow
question_answer 274) A perpendicular to the pont P(1, 0,3) is drawn to the line joining the points A (4,7,1) and B(3,5,3). The foot of perpendicular to the line is
A)
\[(5,7,1)\]
done
clear
B)
\[\left( \frac{5}{3},\frac{7}{3},\frac{17}{3} \right)\]
done
clear
C)
\[\left( \frac{7}{3},\frac{5}{3},\frac{7}{3} \right)\]
done
clear
D)
\[\left( \frac{5}{2},\frac{2}{3},\frac{7}{3} \right)\]
done
clear
View Answer play_arrow
question_answer 275) If the coefficient of\[{{x}^{7}}\]and\[{{x}^{8}}\]in the expansion of\[{{\left( 2+\frac{x}{3} \right)}^{n}}\]are equal, then n is equal to
A)
55
done
clear
B)
56
done
clear
C)
45
done
clear
D)
15
done
clear
View Answer play_arrow
question_answer 276) Sum of the\[n\]terms of the series \[\frac{3}{{{1}^{2}}}+\frac{5}{{{1}^{2}}+{{2}^{2}}}+\frac{7}{{{1}^{2}}+{{2}^{2}}+{{3}^{2}}}+....\]is
A)
\[\frac{4n}{(n+1)}\]
done
clear
B)
\[\frac{6n}{(n+1)}\]
done
clear
C)
\[\frac{12n}{(n+1)}\]
done
clear
D)
\[\frac{9n}{(n+1)}\]
done
clear
View Answer play_arrow
question_answer 277) If difference of the roots of the equation \[{{x}^{2}}+px+8=0\]is 2, then value of p is
A)
\[\pm 6\]
done
clear
B)
\[\pm 2\]
done
clear
C)
\[2,-6\]
done
clear
D)
\[6,2\]
done
clear
View Answer play_arrow
question_answer 278) If the roots of the equation\[\frac{{{x}^{2}}-bx}{ax-c}=\frac{\lambda -1}{\lambda +1}\]are equal and opposite in sign, then\[\lambda \]is equal to
A)
\[\frac{a+b}{a-b}\]
done
clear
B)
\[\frac{a-b}{a+b}\]
done
clear
C)
0
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 279) \[\int{\left( \frac{a{{x}^{3}}+b{{x}^{2}}+c}{{{x}^{4}}} \right)}dx\]is equal to
A)
\[a\log x+\frac{b}{{{x}^{3}}}+\frac{c}{3{{x}^{3}}}+A\]
done
clear
B)
\[a\log x+\frac{b}{x}-\frac{c}{3{{x}^{3}}}+A\]
done
clear
C)
\[a\log x-\frac{b}{x}-\frac{c}{3{{x}^{3}}}+A\]
done
clear
D)
\[a\log x+\frac{b}{x}+\frac{c}{3{{x}^{3}}}+A\]
done
clear
View Answer play_arrow
question_answer 280) \[\int{\sqrt{{{x}^{2}}+{{a}^{2}}}}dx\]is equal to
A)
\[\frac{x}{2}\sqrt{{{x}^{2}}+{{a}^{2}}}+\frac{{{a}^{2}}}{2}\log \{x+\sqrt{{{x}^{2}}+{{a}^{2}}}\}+c\]
done
clear
B)
\[\frac{x}{2}\sqrt{{{x}^{2}}+{{a}^{2}}}-\frac{{{a}^{2}}}{2}\log \{x+\sqrt{{{x}^{2}}+{{a}^{2}}}\}+c\]
done
clear
C)
\[\frac{x}{2}+\sqrt{{{x}^{2}}+{{a}^{2}}}+\frac{{{a}^{2}}}{2}\log \{x-\sqrt{{{x}^{2}}+{{a}^{2}}}\}+c\]
done
clear
D)
\[\sqrt{{{x}^{2}}+{{a}^{2}}}+\frac{{{a}^{2}}}{2}\log \{x-\sqrt{{{x}^{2}}+{{a}^{2}}}\}+c\]
done
clear
View Answer play_arrow
question_answer 281) If 6 points out of 10 points are collinear, then number of triangles made by using these 10 points is
A)
100
done
clear
B)
16
done
clear
C)
12
done
clear
D)
14
done
clear
View Answer play_arrow
question_answer 282) If\[{{a}_{ij}}=\frac{1}{2}2i-3j,\]then matrix\[{{A}_{2\times 2}}=[{{a}_{ij}}]\]is equal to
A)
\[\left[ \begin{matrix} 1/2 & 2 \\ 1/2 & 1 \\ \end{matrix} \right]\]
done
clear
B)
\[\left[ \begin{matrix} 2 & 2 \\ 1/2 & 1/2 \\ \end{matrix} \right]\]
done
clear
C)
\[\left[ \begin{matrix} 1/2 & -2 \\ -1/2 & 1 \\ \end{matrix} \right]\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 283) If\[A=\left[ \begin{matrix} 1 & -2 & 1 \\ 2 & 1 & 3 \\ \end{matrix} \right]\]and\[B=\left[ \begin{matrix} 2 & 1 \\ 3 & 2 \\ 1 & 1 \\ \end{matrix} \right],\]then\[{{(AB)}^{T}}\]is equal to
A)
\[{{A}^{T}}{{B}^{T}}\]
done
clear
B)
\[{{B}^{T}}{{A}^{T}}\]
done
clear
C)
\[{{(AB)}^{-1}}\]
done
clear
D)
\[A.B\]
done
clear
View Answer play_arrow
question_answer 284) Probabilities to solve a problem by three students A, B and C are 1/2, 1/3 and 1/4, then the probability that the problem will be solved, is
A)
1/2
done
clear
B)
1/4
done
clear
C)
3/4
done
clear
D)
2/3
done
clear
View Answer play_arrow
question_answer 285) Two cards are drawn with replacement from a well shuffled pack of 52 cards. The probability that both are ace, is
A)
4/13
done
clear
B)
1/169
done
clear
C)
2/13
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 286) Length of normal at any point of the curve\[x=a(t+\sin t),y=a(1-\cos t),\]is
A)
\[a\sin t\]
done
clear
B)
\[2a\sin {{t}^{3}}\left( \frac{t}{2} \right)\sec \left( \frac{t}{2} \right)\]
done
clear
C)
\[2a\sin \left( \frac{t}{2} \right)\tan \left( \frac{t}{2} \right)\]
done
clear
D)
\[2a\sin \left( \frac{t}{2} \right)\]
done
clear
View Answer play_arrow
question_answer 287) \[\underset{x\to 0}{\mathop{\lim }}\,{{\left[ \tan \left( \frac{\pi }{4}+x \right) \right]}^{1/x}}\]is equal to
A)
\[{{e}^{2}}\]
done
clear
B)
e
done
clear
C)
\[{{e}^{-1}}\]
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 288) \[^{x}{{C}_{r}}{{+}^{x}}{{C}_{r-1}}^{y}{{C}_{1}}{{+}^{x}}{{C}_{r-2}}^{y}{{C}_{2}}+{{.....}^{y}}{{C}_{r}}\]is equal to
A)
\[\frac{x+y!}{r!}\]
done
clear
B)
\[^{x+y}{{C}_{r}}^{xy}{{C}_{r}}\]
done
clear
C)
\[^{x+y}{{C}_{r}}\]
done
clear
D)
\[^{xy}{{C}_{r}}\]
done
clear
View Answer play_arrow
question_answer 289) If \[A=\left[ \begin{matrix} 3 & 8 \\ 2 & 1 \\ \end{matrix} \right],\]then\[{{A}^{-1}}\]is equal to
A)
\[-\frac{1}{13}\left[ \begin{matrix} 1 & 7 \\ 8 & 3 \\ \end{matrix} \right]\]
done
clear
B)
\[\left[ \begin{matrix} -1 & 8 \\ 2 & -3 \\ \end{matrix} \right]\]
done
clear
C)
\[-\frac{1}{13}\left[ \begin{matrix} 1 & -8 \\ -2 & 3 \\ \end{matrix} \right]\]
done
clear
D)
does not exist
done
clear
View Answer play_arrow
question_answer 290) Direction ratios of two lines are\[5,-12,13\]and \[-3,4,5,\]then angle between the lines is
A)
\[{{\cos }^{-1}}\left( \frac{2}{65} \right)\]
done
clear
B)
\[{{\cos }^{-1}}\left( \frac{1}{65} \right)\]
done
clear
C)
\[{{\cos }^{-1}}\left( \frac{3}{65} \right)\]
done
clear
D)
\[\frac{\pi }{3}\]
done
clear
View Answer play_arrow
question_answer 291) In a GP, the ratio of the sum of first three terms and first six terms is 125:152, then common ratio is
A)
\[\frac{1}{5}\]
done
clear
B)
\[\frac{2}{5}\]
done
clear
C)
\[\frac{3}{5}\]
done
clear
D)
\[\frac{4}{5}\]
done
clear
View Answer play_arrow
question_answer 292) Triangle made by the points\[(0,7,10),(-1,6,6)\]and\[(-4,9,6)\]is
A)
right angled
done
clear
B)
isosceles
done
clear
C)
equilateral
done
clear
D)
right angled isosceles
done
clear
View Answer play_arrow
question_answer 293) If the equation\[a(b-c){{x}^{2}}+b(c-a)x+\]\[c(a-b)=0\]has equal roots, then a, b, c are in
A)
AP
done
clear
B)
HP
done
clear
C)
GP
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 294) If P=0.25, P=0.5 and\[P(A\cap B)=0.14,\]then\[P(\overline{A}\cap \overline{B})\]is equal to
A)
0.39
done
clear
B)
0.61
done
clear
C)
0.48
done
clear
D)
0.45
done
clear
View Answer play_arrow
question_answer 295) If\[y=\sqrt{\sin x+y},\]then\[\frac{dy}{dx}\]is equal to
A)
\[\frac{\cos x}{2y-1}\]
done
clear
B)
\[\frac{\sin x}{2y-1}\]
done
clear
C)
\[\frac{\log x}{1-2y}\]
done
clear
D)
\[\frac{\sin x}{1+xy}\]
done
clear
View Answer play_arrow
question_answer 296) Three vertices of a parallelogram are\[A(-1,-2),\]\[B(1,0)\]and\[C(3,4),\]then the fourth vertex is
A)
(1, 2)
done
clear
B)
\[(-3,-4)\]
done
clear
C)
(2, 0)
done
clear
D)
\[(-1,2)\]
done
clear
View Answer play_arrow
question_answer 297) If\[2x-\left[ \begin{matrix} 1 & 2 \\ 7 & 4 \\ \end{matrix} \right]=\left[ \begin{matrix} 3 & 2 \\ 0 & -2 \\ \end{matrix} \right],\]then\[x\]is equal to
A)
\[\left[ \begin{matrix} 4 & 4 \\ 7 & 2 \\ \end{matrix} \right]\]
done
clear
B)
\[\left[ \begin{matrix} 2 & 2 \\ 7/2 & 1 \\ \end{matrix} \right]\]
done
clear
C)
\[\left[ \begin{matrix} 3 & -1 \\ 7/2 & 2 \\ \end{matrix} \right]\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 298) \[\int{\frac{{{x}^{2}}}{{{x}^{2}}+4}}\]is equal to
A)
\[x-4{{\tan }^{-1}}\left( \frac{x}{2} \right)+c\]
done
clear
B)
\[x-2{{\tan }^{-1}}\left( \frac{x}{2} \right)+c\]
done
clear
C)
\[x+4{{\tan }^{-1}}\left( \frac{x}{2} \right)+c\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 299) The function \[f(x)=sin\left( \frac{\pi x}{2} \right)+2cos\left( \frac{\pi x}{3} \right)-\tan \left( \frac{\pi x}{4} \right)\]is a periodic function. It's period is
A)
6
done
clear
B)
3
done
clear
C)
4
done
clear
D)
12
done
clear
View Answer play_arrow
question_answer 300) Line\[ax+by+c=0\]is normal to the circle\[{{x}^{2}}+{{y}^{2}}={{r}^{2}}\]. Length of the intercepts cut by the line on the circle is
A)
r
done
clear
B)
\[{{r}^{2}}\]
done
clear
C)
2r
done
clear
D)
\[\sqrt{r}\]
done
clear
View Answer play_arrow