question_answer 1) If force\[(F)\], work \[(W)\] and velocity \[(V)\] are taken as fundamental quantities, then the dimensional formula of time\[(T)\]is
A)
\[[WFv]\]
done
clear
B)
\[[WF{{v}^{-1}}]\]
done
clear
C)
\[[{{W}^{-1}}{{F}^{-1}}v]\]
done
clear
D)
\[[W{{F}^{-1}}{{v}^{-1}}]\]
done
clear
View Answer play_arrow
question_answer 2) Given two vectors \[\mathbf{\vec{A}}=-\mathbf{\hat{i}}+2\mathbf{\hat{j}}-3\mathbf{\hat{k}}\] and\[\mathbf{\vec{B}}=4\mathbf{\hat{i}}-2\mathbf{\hat{j}}+6\mathbf{\hat{k}}\]. The angle made by\[(\mathbf{\vec{A}}+\mathbf{\vec{B}})\]with \[x-\]axis is
A)
\[{{30}^{o}}\]
done
clear
B)
\[{{45}^{o}}\]
done
clear
C)
\[{{60}^{o}}\]
done
clear
D)
\[{{90}^{o}}\]
done
clear
View Answer play_arrow
question_answer 3) A body is thrown vertically up with certain initial velocity. The potential and kinetic energies of the body are equal at a point P in its path. If the same body is thrown with double the velocity upwards, the ratio of potential and kinetic energies of the body when it crosses the same point, is
A)
\[1:1\]
done
clear
B)
\[1:4\]
done
clear
C)
\[1:7\]
done
clear
D)
\[1:8\]
done
clear
View Answer play_arrow
question_answer 4) A body of mass \[2kg\] is thrown up vertically with kinetic energy of\[490J\]. If \[g=9.8\text{ }m/{{s}^{2}}\], the height at which the kinetic energy of the body becomes half of the original value, is
A)
\[50m\]
done
clear
B)
\[25m\]
done
clear
C)
\[12.5m\]
done
clear
D)
\[19.6m\]
done
clear
View Answer play_arrow
question_answer 5) The apparent weight of a person inside a lift is \[{{w}_{1}}\] when lift moves up with a certain acceleration and is \[{{w}_{2}}\] when lift moves down with same acceleration. The weight of the person when lift moves up with constant speed is
A)
\[\frac{{{w}_{1}}+{{w}_{2}}}{2}\]
done
clear
B)
\[\frac{{{w}_{1}}-{{w}_{2}}}{2}\]
done
clear
C)
\[2{{w}_{1}}\]
done
clear
D)
\[2{{w}_{2}}\]
done
clear
View Answer play_arrow
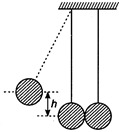
question_answer 6)
In the figure, pendulum bob on left side is pulled aside to a height h from its initial position. After it is released it collides with the right pendulum bob at rest, which is of same mass. After the collision the two bobs stick together and raise to a height
A)
\[\frac{3h}{4}\]
done
clear
B)
\[\frac{2h}{3}\]
done
clear
C)
\[\frac{h}{2}\]
done
clear
D)
\[\frac{h}{4}\]
done
clear
View Answer play_arrow
question_answer 7) A sphere of mass m moving with constant velocity\[u\], collides with another stationary sphere of same mass. If e is the coefficient of restitution, the ratio of the final velocities of the first and second spheres is
A)
\[\frac{1+e}{1-e}\]
done
clear
B)
\[\frac{1-e}{1+e}\]
done
clear
C)
\[\frac{e}{1-e}\]
done
clear
D)
\[\frac{1+e}{e}\]
done
clear
View Answer play_arrow
question_answer 8) A block of mass \[2kg\] is placed on the surface of a trolley of mass \[20kg\] which is on a smooth surface. The coefficient of friction between the block and the surface of the trolley is\[0.25\]. If a horizontal force of \[2N\] acts on the block, the acceleration of the system in \[m{{s}^{-2}}\] is\[(g=10m{{s}^{-2}})\]
A)
\[1.8\]
done
clear
B)
\[1.0\]
done
clear
C)
\[0.9\]
done
clear
D)
\[0.09\]
done
clear
View Answer play_arrow
question_answer 9) The radius of gyration of a rod of length \[L\] and mass \[M\] about an axis perpendicular to its length and passing through a point at a distance \[L/3\] from one of its ends is
A)
\[\frac{\sqrt{7}}{6}L\]
done
clear
B)
\[\frac{{{L}^{2}}}{9}\]
done
clear
C)
\[\frac{L}{3}\]
done
clear
D)
\[\frac{\sqrt{5}}{2}L\]
done
clear
View Answer play_arrow
question_answer 10) A ball of mass \[0.6kg\] attached to a light inextensible string rotates in a vertical circle of radius \[0.75m\] such that it has a speed of\[5m{{s}^{-1}}\] when the string is horizontal. Tension in string when it is horizontal on other side is\[(g=10m{{s}^{-2}})\]
A)
\[30N\]
done
clear
B)
\[26N\]
done
clear
C)
\[20N\]
done
clear
D)
\[6N\]
done
clear
View Answer play_arrow
question_answer 11) A body of mass m is raised from the surface of the earth to a height nR (R = radius of earth). Magnitude of the change in the gravitational potential energy of the body is (\[g=\] acceleration due to gravity on the surface of earth)
A)
\[\left( \frac{n}{n+1} \right)mgR\]
done
clear
B)
\[\left( \frac{n-1}{n} \right)mgR\]
done
clear
C)
\[\left( \frac{mgR}{n} \right)\]
done
clear
D)
\[\frac{mgR}{(n-1)}\]
done
clear
View Answer play_arrow
question_answer 12) The displacement of a particle of mass\[3g\] executing simple harmonic motion is given by \[Y=3\,\,\sin (0.2t)\] in SI units. The kinetic energy of the particle at a point which is at a distance equal to \[\frac{1}{3}\] of its amplitude from its mean position is
A)
\[12\times {{10}^{3}}J\]
done
clear
B)
\[25\times {{10}^{-3}}J\]
done
clear
C)
\[0.48\times {{10}^{-3}}J\]
done
clear
D)
\[0.24\times {{10}^{-3}}J\]
done
clear
View Answer play_arrow
question_answer 13) A body subjected to strain several times will not obey Hookes law due to
A)
yield point
done
clear
B)
permanent state
done
clear
C)
elastic fatigue
done
clear
D)
breaking stress
done
clear
View Answer play_arrow
question_answer 14) A liquid drop of radius \[R\] breaks into 64 tiny drops each of radius\[r\]. If the surface tension of the liquid is \[T\], then the gain in energy is
A)
\[48\pi {{R}^{2}}T\]
done
clear
B)
\[12\pi {{r}^{2}}T\]
done
clear
C)
\[96\pi {{R}^{2}}T\]
done
clear
D)
\[192\pi {{r}^{2}}T\]
done
clear
View Answer play_arrow
question_answer 15) The pressure on the top surface of an aeroplane wing is \[0.8\times {{10}^{5}}\] Pa and the pressure on the bottom surface is \[0.75\times {{10}^{5}}\] Pa. If the area of each surface is\[50\,\,{{m}^{2}}\], the dynamic lift on the wing is
A)
\[25\times {{10}^{4}}N\]
done
clear
B)
\[0.5\times {{10}^{4}}N\]
done
clear
C)
\[5\times {{10}^{4}}N\]
done
clear
D)
\[0.25\times {{10}^{5}}N\]
done
clear
View Answer play_arrow
question_answer 16) Two rods of different materials with coefficients of linear thermal expansion\[{{\alpha }_{1}},\,\,{{\alpha }_{2}}\]and Youngs moduli \[{{Y}_{1}}\] and \[{{Y}_{2}}\] respectively are fixed between two rigid walls. They are heated to have the same increase in temperature. If the rods do not bend and if\[{{\alpha }_{1}}:{{\alpha }_{2}}=2:3\], then the thermal stresses developed in the two rods will be equal when \[{{Y}_{1}}:{{Y}_{2}}\] is equal to
A)
\[2:3\]
done
clear
B)
\[2:5\]
done
clear
C)
\[3:2\]
done
clear
D)
\[5:2\]
done
clear
View Answer play_arrow
question_answer 17) What fraction of the volume of a glass flask must be filled with mercury so that the volume of the empty space may be the same at all temperatures? \[({{\alpha }_{glass}}=9\times {{10}^{-6}}/{}^\circ C,\,\,{{\gamma }_{Hg}}=18.9\times {{10}^{-5}}/{}^\circ C)\]
A)
\[\frac{1}{2}\]
done
clear
B)
\[\frac{1}{7}\]
done
clear
C)
\[\frac{1}{4}\]
done
clear
D)
\[\frac{1}{5}\]
done
clear
View Answer play_arrow
question_answer 18) For an adiabatic process, the relation between\[V\] and \[T\] is given by
A)
\[T{{V}^{\gamma }}=\]constant
done
clear
B)
\[{{T}^{\gamma }}V=\]constant
done
clear
C)
\[T{{V}^{1-\gamma }}=\]constant
done
clear
D)
\[T{{V}^{\gamma -1}}=\]constant
done
clear
View Answer play_arrow
question_answer 19) Consider the following two statements and choose the correct answer. (a) If heat is added to a system its temperature must always increase. (b) If positive work is done by a system in thermodynamic process, its volume must increase.
A)
Both (a) and (b) are correct
done
clear
B)
(A) is correct, but (B) is wrong
done
clear
C)
(B) is correct, but (A) is wrong
done
clear
D)
Both (A) and (B) are wrong
done
clear
View Answer play_arrow
question_answer 20) The power of a black body at temperature \[200K\] is\[544W\]. Its surface area is\[(\sigma =5.67\times {{10}^{-8}}W{{m}^{2}}{{K}^{-4}})\]
A)
\[6\times {{10}^{-2}}{{m}^{2}}\]
done
clear
B)
\[6{{m}^{2}}\]
done
clear
C)
\[6\times {{10}^{-6}}{{m}^{2}}\]
done
clear
D)
\[6\times {{10}^{2}}{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 21) A uniform wire of linear density \[0.004kg\text{-}{{m}^{-1}}\] when stretched between two rigid supports with a tension \[3.6\times {{10}^{2}}N\] resonates with a frequency of\[420Hz\]. The next harmonic frequency with which the wire resonates is\[490Hz\]. The length of the wire in metres is
A)
\[1.41\]
done
clear
B)
\[2.14\]
done
clear
C)
\[2.41\]
done
clear
D)
\[3.14\]
done
clear
View Answer play_arrow
question_answer 22) To increase the frequency by\[20%\], the tension in the string vibrating on a sonometer has to be increased by
A)
\[44%\]
done
clear
B)
\[33%\]
done
clear
C)
\[22%\]
done
clear
D)
\[11%\]
done
clear
View Answer play_arrow
question_answer 23) In Ramsden eye-piece the focal length of each lens is\[F\]. The distance of the image formed by the objective lens from the eye lens is
A)
\[\frac{14F}{15}\]
done
clear
B)
\[\frac{13F}{14}\]
done
clear
C)
\[\frac{12F}{13}\]
done
clear
D)
\[\frac{11F}{12}\]
done
clear
View Answer play_arrow
question_answer 24) The velocities of light in two different mediums are \[2\times {{10}^{8}}m{{s}^{-1}}\] and \[2.5\times {{10}^{8}}m{{s}^{-1}}\] respectively. The critical angle for these mediums is
A)
\[{{\sin }^{-1}}\left( \frac{1}{5} \right)\]
done
clear
B)
\[{{\sin }^{-1}}\left( \frac{4}{5} \right)\]
done
clear
C)
\[{{\sin }^{-1}}\left( \frac{1}{2} \right)\]
done
clear
D)
\[{{\sin }^{-1}}\left( \frac{1}{4} \right)\]
done
clear
View Answer play_arrow
question_answer 25) A diverging meniscus lens of \[1.5\] refractive index has concave surfaces of radii \[3\] and\[4cm.\]The position of the image, if an object is placed \[12cm\] in front of the lens, is
A)
\[7cm\]
done
clear
B)
\[-8cm\]
done
clear
C)
\[9cm\]
done
clear
D)
\[10cm\]
done
clear
View Answer play_arrow
question_answer 26) The source is at some distance from an obstacle. Distance between obstacle and the point of observation is \[b\] and wavelength of light is\[\lambda \]. Then the average distance of nth Fresnel zone will be at a distance ... from the point of observation.
A)
\[\frac{bn\,\lambda }{2}\]
done
clear
B)
\[b-\frac{n\,\lambda }{2}\]
done
clear
C)
\[b+\frac{n\,\lambda }{2}\]
done
clear
D)
\[b-n\,\lambda \]
done
clear
View Answer play_arrow
question_answer 27) A bar magnet suspended freely in a uniform magnetic field is vibrating with a time period of\[3s\]. If the field strength is increased to\[4\] times of the earlier field strength, the time period will be (in seconds)
A)
12
done
clear
B)
6
done
clear
C)
1.5
done
clear
D)
0.75
done
clear
View Answer play_arrow
question_answer 28) A bar magnet of magnetic moment \[{{M}_{1}}\] is axially cut into two equal parts. If these two pieces are arranged perpendicular to each other, the resultant magnetic moment is\[{{M}_{2}}\]. Then the value of\[{{M}_{1}}/{{M}_{2}}\]is
A)
\[\frac{1}{2\sqrt{2}}\]
done
clear
B)
\[1\]
done
clear
C)
\[\frac{1}{\sqrt{2}}\]
done
clear
D)
\[\sqrt{2}\]
done
clear
View Answer play_arrow
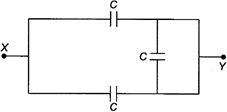
question_answer 29)
The equivalent capacity between the points\[X\]and\[Y\]in the circuit with\[C=1\mu F\]is
A)
\[2\mu F\]
done
clear
B)
\[3\mu F\]
done
clear
C)
\[1\mu F\]
done
clear
D)
\[0.5\mu F\]
done
clear
View Answer play_arrow
question_answer 30) Three charges \[1\mu C,\,\,1\mu C\] and \[2\mu C\] are kept at the vertices \[A,\,\,B\] and \[C\] of an equilateral triangle \[ABC\] of \[10cm\] side, respectively. The resultant force on the charge at \[C\] is
A)
\[0.9N\]
done
clear
B)
\[1.8N\]
done
clear
C)
\[2.72N\]
done
clear
D)
\[3.6N\]
done
clear
View Answer play_arrow
question_answer 31) Two unknown resistances \[X\] and \[Y\] are connected to left and right gaps of a metre bridge and the balancing point is obtained at \[80cm\] from left. When a \[10\,\,\Omega \] resistance is connected in parallel to \[X\] the balancing point is \[50cm\] from left. The values of \[X\] and \[Y\] respectively are
A)
\[40\Omega ,\,\,9\Omega \]
done
clear
B)
\[30\Omega ,\,\,7.5\Omega \]
done
clear
C)
\[20\Omega ,\,\,6\Omega \]
done
clear
D)
\[10\Omega ,\,\,3\Omega \]
done
clear
View Answer play_arrow
question_answer 32) The current in a circuit containing a battery connected to \[2\Omega \] resistance is\[0.9A\]. When a resistance of \[7\Omega \] is connected to the same battery, the current observed in the circuit is\[0.3A\]. Then the internal resistance of the battery is
A)
\[0.1\Omega \]
done
clear
B)
\[0.5\Omega \]
done
clear
C)
\[1\Omega \]
done
clear
D)
\[zero\]
done
clear
View Answer play_arrow
question_answer 33) In a thermo-couple the cold junction is at\[{{30}^{o}}C.\] The temperature of inversion is found to be\[{{540}^{o}}C\], then the neutral temperature is
A)
\[{{270}^{o}}C\]
done
clear
B)
\[{{510}^{o}}C\]
done
clear
C)
\[{{285}^{o}}C\]
done
clear
D)
\[{{240}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 34) The emf induced in a secondary coil is \[20000\,\,V\] when the current breaks in the primary coil. The mutual inductance is \[5H\] and the current reaches to zero in \[{{10}^{-4}}s\] in the primary. The maximum current in the primary before it breaks is
A)
\[0.1A\]
done
clear
B)
\[0.4A\]
done
clear
C)
\[0.6A\]
done
clear
D)
\[0.8A\]
done
clear
View Answer play_arrow
question_answer 35) Two coils are wound on the same iron rod so that the flux generated by one passes through the other. The primary coil has \[{{N}_{p}}\] turns in it and when a current \[2A\] flows through it the flux in it is\[2.5\times {{10}^{-4}}Wb\]. If the secondary coil has 12 turns the mutual inductance of the coils is (assume the secondary coil is in open circuit)
A)
\[10\times {{10}^{-4}}H\]
done
clear
B)
\[15\times {{10}^{-4}}H\]
done
clear
C)
\[20\times {{10}^{-4}}H\]
done
clear
D)
\[25\times {{10}^{-4}}H\]
done
clear
View Answer play_arrow
question_answer 36) A coil of 40 H inductance is connected in series with a resistance of \[8\Omega \] and this combination is connected to the terminals of a \[2V\] battery. The inductive time constant of the circuit is (in second)
A)
40
done
clear
B)
20
done
clear
C)
5
done
clear
D)
0.2
done
clear
View Answer play_arrow
question_answer 37) The velocity of the most energetic electrons emitted from a metallic surface is doubled when the frequency \[v\] of the incident radiation is doubled. The work function of this metal is
A)
\[\frac{hv}{4}\]
done
clear
B)
\[\frac{hv}{3}\]
done
clear
C)
\[\frac{hv}{2}\]
done
clear
D)
\[\frac{2hv}{3}\]
done
clear
View Answer play_arrow
question_answer 38) A proton and an alpha particle are accelerated through the same potential difference. The ratio of the wavelengths associated with proton and alpha particle respectively is
A)
\[1:2:\sqrt{2}\]
done
clear
B)
\[2:1\]
done
clear
C)
\[2:\sqrt{2}:1\]
done
clear
D)
\[4:1\]
done
clear
View Answer play_arrow
question_answer 39) Electron belongs to which of the following class of elementary particles
A)
Hardon
done
clear
B)
Lepton
done
clear
C)
Boson
done
clear
D)
Baryon
done
clear
View Answer play_arrow
question_answer 40) In a transistor circuit the base current changes from \[30\mu A\] to\[90\mu A\]. If the current gain of the transistor is 30, the change in the collector current is
A)
\[4\,\,mA\]
done
clear
B)
\[2\,\,mA\]
done
clear
C)
\[3.6\,\,mA\]
done
clear
D)
\[1.8\,\,mA\]
done
clear
View Answer play_arrow
question_answer 41) A man slides down on a telegraphic pole with an acceleration equal to one-fourth of acceleration due to gravity. The frictional force between man and pole is equal to in terms of mans weight\[w\]
A)
\[\frac{w}{4}\]
done
clear
B)
\[\frac{w}{2}\]
done
clear
C)
\[\frac{3w}{4}\]
done
clear
D)
\[w\]
done
clear
View Answer play_arrow
question_answer 42) The magnitude of maximum acceleration is \[\pi \] times that of maximum velocity of a simple harmonic oscillator. The time period of the oscillator in second is
A)
4
done
clear
B)
2
done
clear
C)
1
done
clear
D)
0.5
done
clear
View Answer play_arrow
question_answer 43) A liquid does not wet the solid surface if the angle of contact is
A)
Zero
done
clear
B)
equal to\[{{45}^{o}}\]
done
clear
C)
equal to\[{{90}^{o}}\]
done
clear
D)
greater than\[{{90}^{o}}\]
done
clear
View Answer play_arrow
question_answer 44) A clock which keeps correct time at\[{{20}^{o}}C\], is subjected to\[{{40}^{o}}C\]. If coefficient of linear expansion of the pendulum is\[12\times {{10}^{-6}}{{/}^{o}}C\]. How much will it gain or lose time?
A)
10.3 s/day
done
clear
B)
20.6 s/day
done
clear
C)
5 s/day
done
clear
D)
20 min/day
done
clear
View Answer play_arrow
question_answer 45) Two gases \[A\] and \[B\] having same pressure\[P\], volume \[V\] and absolute temperature \[T\] are mixed. If the mixture has the volume and temperature as \[V\] and \[T\] respectively, then the pressure of the mixture is
A)
\[2\,\,P\]
done
clear
B)
\[P\]
done
clear
C)
\[\frac{P}{2}\]
done
clear
D)
\[4P\]
done
clear
View Answer play_arrow
question_answer 46) The temperature of the system decreases in the process of
A)
free expansion
done
clear
B)
adiabatic expansion
done
clear
C)
isothermal expansion
done
clear
D)
isothermal compression
done
clear
View Answer play_arrow
question_answer 47) In Ramsden eyepiece, the two planoconvex lenses each of focal length \[f\] are separated by a distance\[12cm\]. The equivalent focal length (in \[cm\]) of the eyepiece is
A)
10.5
done
clear
B)
12.0
done
clear
C)
13.5
done
clear
D)
15.5
done
clear
View Answer play_arrow
question_answer 48) In Huygens eyepiece
A)
the cross wires are outside the eyepiece
done
clear
B)
condition for achromatism is satisfied
done
clear
C)
condition for minimum spherical aberration is not satisfied
done
clear
D)
the image formed by the objective is a virtual image
done
clear
View Answer play_arrow
question_answer 49) The natural frequency of an\[L-C\] circuit is \[1,25,000\] cycle/s. Then the capacitor \[C\] is replaced by another capacitor with a dielectric medium of dielectric constant\[K\]. In this case, the frequency decreases by\[25kHz\]. The value of \[K\] is
A)
3.0
done
clear
B)
2.1
done
clear
C)
1.56
done
clear
D)
1.7
done
clear
View Answer play_arrow
question_answer 50) In Sun, the important source of energy is
A)
proton-proton cycle
done
clear
B)
carbon-nitrogen cycle
done
clear
C)
carbon-carbon cycle
done
clear
D)
nitrogen-nitrogen cycle
done
clear
View Answer play_arrow
question_answer 51) Calculate the difference between \[\Delta E\] and \[\Delta H\]for the following reaction at \[{{27}^{o}}C\](in kcal). \[{{C}_{(graphite)}}+{{H}_{2}}(g)\xrightarrow{{}}C{{H}_{4}}(g)\]
A)
\[-0.6\]
done
clear
B)
\[-1.2\]
done
clear
C)
\[+0.6\]
done
clear
D)
\[+1.2\]
done
clear
View Answer play_arrow
question_answer 52) DNA multiplication is called
A)
translation
done
clear
B)
transduction
done
clear
C)
transcription
done
clear
D)
replication
done
clear
View Answer play_arrow
question_answer 53) \[{{C}_{2}}{{H}_{5}}Cl\xrightarrow[A{{g}_{2}}O]{Moist}A\xrightarrow[{{360}^{o}}C]{A{{l}_{2}}{{O}_{3}}}B\xrightarrow[{}]{{{S}_{2}}C{{l}_{2}}}\] In the above sequence of reactions, identify\[C.\]
A)
chloretone
done
clear
B)
chloropicrin
done
clear
C)
mustard gas
done
clear
D)
lewisite gas
done
clear
View Answer play_arrow
question_answer 54) \[SiC{{l}_{4}}\xrightarrow{{{H}_{2}}O}X\xrightarrow{{{1000}^{o}}C}Y\] In the above reaction, \[X\]and \[Y\] respectively, are
A)
\[Si{{O}_{2}}\]and\[Si\]
done
clear
B)
\[{{H}_{4}}Si{{O}_{4}}\]and\[Si{{O}_{2}}\]
done
clear
C)
\[{{H}_{2}}SiC{{l}_{6}}\]and\[Si{{O}_{2}}\]
done
clear
D)
\[{{H}_{4}}Si{{O}_{4}}\]and\[Si\]
done
clear
View Answer play_arrow
question_answer 55) What is the wave number of 4th line in Balmer series of hydrogen spectrum? \[(R=1,09,677c{{m}^{-1}})\]
A)
\[24,630c{{m}^{-1}}\]
done
clear
B)
\[24,360c{{m}^{-1}}\]
done
clear
C)
\[24,730c{{m}^{-1}}\]
done
clear
D)
\[24,372c{{m}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 56) Disperse phase and dispersion medium in butter are respectively
A)
solid and liquid
done
clear
B)
liquid and solid
done
clear
C)
liquid and liquid
done
clear
D)
solid and solid
done
clear
View Answer play_arrow
question_answer 57) When oxyhaemoglobin changes to de-oxyhaenioglobin, \[F{{e}^{2+}}\]ion changes from
A)
diamagnetic to paramagnetic
done
clear
B)
paramagnetic to diamagnetic
done
clear
C)
diamagnetic to ferromagnetic
done
clear
D)
paramagnetic to ferromagnetic
done
clear
View Answer play_arrow
question_answer 58) \[A\]react with \[{{C}_{2}}{{H}_{5}}I\] giving \[B\] and\[Nal\]. Here\[A\]and \[B\] respectively, are
A)
\[C{{H}_{3}}COONa,\,\,C{{H}_{3}}OC{{H}_{3}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}},\,\,{{C}_{2}}{{H}_{5}}COO{{C}_{2}}{{H}_{5}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}ONa,\,\,{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}OH,\,\,{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 59) Nitration of aniline is achieved by
A)
direct treatment with nitration mixture under reflux
done
clear
B)
using fuming \[HN{{O}_{3}}\]
done
clear
C)
acetylation followed by nitration and subsequent hydrolysis
done
clear
D)
\[KN{{O}_{3}}+conc\,\,HN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 60) When\[AgN{{O}_{3}}\]solution is added in excess to \[1\,\,M\] solution of\[CoC{{l}_{3}}\cdot xN{{H}_{3}}\], one mole of \[AgCl\] is formed. What is the value of\[x\]?
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 61) Which of the following is the decreasing order of boiling points of\[V\]group hydrides?
A)
\[N{{H}_{3}}>P{{H}_{3}}>As{{H}_{3}}>Sb{{H}_{3}}\]
done
clear
B)
\[Sb{{H}_{3}}>As{{H}_{3}}>P{{H}_{3}}>N{{H}_{3}}\]
done
clear
C)
\[P{{H}_{3}}>N{{H}_{3}}>As{{H}_{3}}>Sb{{H}_{3}}\]
done
clear
D)
\[Sb{{H}_{3}}>N{{H}_{3}}>As{{H}_{3}}>P{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 62) The atomic number of an element \[M\] is 26. How many electrons are present in the M-shell of the element in its\[{{M}^{3+}}\]state?
A)
11
done
clear
B)
15
done
clear
C)
14
done
clear
D)
13
done
clear
View Answer play_arrow
question_answer 63) \[{{N}_{2}}(g)+3{{H}_{2}}(g)2N{{H}_{3}}(g)+22kcal\]. The activation energy for the forward reaction is 50 kcal. What is the activation energy for the backward reaction?
A)
\[-72kcal\]
done
clear
B)
\[-28kcal\]
done
clear
C)
\[+28kcal\]
done
clear
D)
\[+72kcal\]
done
clear
View Answer play_arrow
question_answer 64) The raw material used in nylon-6 is
A)
adipic acid
done
clear
B)
phthalic acid
done
clear
C)
ethylene glycol
done
clear
D)
caprolactum
done
clear
View Answer play_arrow
question_answer 65) Which one of the following reactions is called Rosemond reaction?
A)
Aldehydes are reduced to alcohols
done
clear
B)
Acids are converted to acid chlorides
done
clear
C)
Alcohols are reduced to hydrocarbons
done
clear
D)
Acid chlorides are reduced to aldehydes
done
clear
View Answer play_arrow
question_answer 66) \[IUPAC\]name of\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{align} & | \\ & C{{H}_{2}} \\ & | \\ & C{{H}_{3}} \\ \end{align}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{2}}\]is
A)
2-methyl-3-ethyl-1-pentene
done
clear
B)
3-ethyl-4-methyl-4-pentene
done
clear
C)
3-ethyl-2-methyl-1-pentene
done
clear
D)
3-methyl-2-ethyl-1-pentene
done
clear
View Answer play_arrow
question_answer 67) In the separation of noble gas mixture from air by Ramsay Rayleighs first method, the substances used for the removal of\[{{N}_{2}}\]and\[{{O}_{2}}\] respectively, are
A)
\[Cu\]and\[Mg\]
done
clear
B)
\[Mg\]and\[Cu\]
done
clear
C)
\[C\]and\[Ca{{C}_{2}}\]
done
clear
D)
\[KOH\]solution
done
clear
View Answer play_arrow
question_answer 68) The fraction of element disintegrated after 4 half-lifes in percentage is
A)
\[75%\]
done
clear
B)
\[87.5%\]
done
clear
C)
\[93.75%\]
done
clear
D)
\[92.5%\]
done
clear
View Answer play_arrow
question_answer 69) The most probable velocity of a gas molecule at \[298K\] is\[300m/s\]. Its RMS velocity, in\[(m/s)\] is
A)
420
done
clear
B)
245
done
clear
C)
402
done
clear
D)
367
done
clear
View Answer play_arrow
question_answer 70) Schottky defect is observed in the crystal of
A)
\[NaCl\]
done
clear
B)
\[TiCl\]
done
clear
C)
\[AgCl\]
done
clear
D)
\[MgC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 71) A mixture of amylose and amylopectin is called
A)
lactose
done
clear
B)
starch
done
clear
C)
cellulose
done
clear
D)
sucrose
done
clear
View Answer play_arrow
question_answer 72) Match the following Set I Set II \[{{C}_{2}}{{H}_{5}}OH\xrightarrow[{{170}^{o}}C]{Con.{{H}_{2}}S{{O}_{4}}}\] 1. Methane \[CH{{I}_{3}}\xrightarrow[Ag(Powder)]{\Delta }\] 2. Ethylene \[\begin{align} & C{{H}_{3}}COONa(aq) \\ & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\xrightarrow{Electrolysis} \\ \end{align}\] 3. Benzene \[C{{H}_{3}}COONa\xrightarrow[CaO\,\,\Delta ]{NaOH}\] 4. Acetylene 5. Ethane The correct match is
A)
\[A-2,\,\,B-4,\,\,C-5,\,\,D-1\]
done
clear
B)
\[A-2,\,\,B-4,\,\,C-5,\,\,D-3\]
done
clear
C)
\[A-4,\,\,B-2,\,\,C-5,\,\,D-1\]
done
clear
D)
\[A-4,\,\,B-2,\,\,C-5,\,\,D-3\]
done
clear
View Answer play_arrow
question_answer 73) One gas bleaches the colour of flowers by reduction and another gas by oxidation. The gases respectively, are
A)
\[S{{O}_{2}}\]and\[C{{l}_{2}}\]
done
clear
B)
\[CO\]and\[C{{l}_{2}}\]
done
clear
C)
\[N{{H}_{3}}\]and\[S{{O}_{2}}\]
done
clear
D)
\[{{H}_{2}}S\]and\[Br\]
done
clear
View Answer play_arrow
question_answer 74) Lanthanide contraction is due to increase in
A)
shielding by 4f electrons
done
clear
B)
atomic number
done
clear
C)
effective nuclear charge
done
clear
D)
sizeof4forbitals
done
clear
View Answer play_arrow
question_answer 75) What is the electrode potential (in\[V\]) of the following electrode at\[{{25}^{o}}C\]? \[N{{i}^{2+}}(0.1M/Ni(s)\](Standard reduction potential of\[N{{i}^{2+}}|Ni\]is\[-0.25V,\,\,\frac{2.303RT}{F}=0.06\]
A)
\[-0.28V\]
done
clear
B)
\[-0.34V\]
done
clear
C)
\[-0.82V\]
done
clear
D)
\[-0.22V\]
done
clear
View Answer play_arrow
question_answer 76) What is the equation for the equilibrium constant\[({{K}_{c}})\]for the following reaction? \[\frac{1}{2}A(g)+\frac{1}{3}(g)\frac{2}{3}C(g)\]
A)
\[{{K}_{c}}=\frac{{{[A]}^{1/2}}{{[B]}^{1/3}}}{{{[C]}^{3/2}}}\]
done
clear
B)
\[{{K}_{c}}=\frac{{{[C]}^{3/2}}}{{{[A]}^{2}}{{[B]}^{3}}}\]
done
clear
C)
\[{{K}_{c}}=\frac{{{[C]}^{2/3}}}{{{[A]}^{1/2}}{{[B]}^{1/3}}}\]
done
clear
D)
\[{{K}_{c}}=\frac{{{[C]}^{2/3}}}{{{[A]}^{1/2}}{{[B]}^{1/3}}}\]
done
clear
View Answer play_arrow
question_answer 77) A drug that is antipyretic as well as analgesic is
A)
chloroquine
done
clear
B)
penicillin
done
clear
C)
paracetamol
done
clear
D)
Chloropromazine hydrochloride
done
clear
View Answer play_arrow
question_answer 78) What is the conjugate base of\[HSO_{4}^{-}\]?
A)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[HSO_{4}^{2-}\]
done
clear
C)
\[SO_{4}^{2-}\]
done
clear
D)
\[{{H}^{+}}\]
done
clear
View Answer play_arrow
question_answer 79) Observe the following reaction\[A(g)+3B(g)\xrightarrow{{}}2C(g)\] The rate of this reaction\[\left( =\frac{d[A]}{dt} \right)\]is \[3\times {{10}^{-3}}\] mol\[{{L}^{-1}}{{\min }^{-1}}\]. What is the value of\[-\frac{d[B]}{dt}\]in mol\[{{L}^{-1}}{{\min }^{-1}}\]?
A)
\[3\times {{10}^{-3}}\]
done
clear
B)
\[9\times {{10}^{-3}}\]
done
clear
C)
\[{{10}^{-3}}\]
done
clear
D)
\[1.5\times {{10}^{-3}}\]
done
clear
View Answer play_arrow
question_answer 80) The bond angle in \[As{{H}_{3}}\] is greater than that in
A)
\[N{{H}_{3}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[BC{{l}_{3}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 81) A \[0.5g/L\] solution of glucose is found to be isotonic with a \[2.5g/L\] solution of an organic compound. What will be the molecular weight of that organic compound?
A)
300
done
clear
B)
600
done
clear
C)
900
done
clear
D)
200
done
clear
View Answer play_arrow
question_answer 82) \[t-\]butyl chloride preferably undergo hydrolysis by
A)
\[{{S}_{N}}1\]mechanism
done
clear
B)
\[{{S}_{N}}2\]mechanism
done
clear
C)
any of (a) and (b)
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 83) Which of the following has lowest boiling point?
A)
\[NaCl\]
done
clear
B)
\[CuCl\]
done
clear
C)
\[CuC{{l}_{2}}\]
done
clear
D)
\[CsCl\]
done
clear
View Answer play_arrow
question_answer 84) To dissolve argentite ore which of the following is used?
A)
\[Na[Ag{{(CN)}_{2}}]\]
done
clear
B)
\[NaCN\]
done
clear
C)
\[NaCl\]
done
clear
D)
\[HCl\]
done
clear
View Answer play_arrow
question_answer 85) The ratio of carbon, hydrogen and oxygen in 2-methyl benzoic acid is
A)
\[4:2:2\]
done
clear
B)
\[2:4:1\]
done
clear
C)
\[4:4:1\]
done
clear
D)
\[4:4:2\]
done
clear
View Answer play_arrow
question_answer 86) The best method to separate the mixture of ortho and para nitrophenol \[(1:1)\] is
A)
vaporisation
done
clear
B)
colour spectrum
done
clear
C)
distillation
done
clear
D)
crystallisation
done
clear
View Answer play_arrow
question_answer 87) Acetylene and \[HCHO\] react in presence of copper acetylide catalyst to form
A)
1-butyne-1, 4-diol
done
clear
B)
2-butyne-1, 2-diol
done
clear
C)
2-butyne-1, 4-diol
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 88) The effective atomic number of\[Cr\]in \[Cr{{(N{{H}_{3}})}_{6}}]C{{l}_{3}}\]is
A)
35
done
clear
B)
36
done
clear
C)
27
done
clear
D)
33
done
clear
View Answer play_arrow
question_answer 89) Which of the following forms colourless compounds?
A)
\[S{{c}^{3+}}\]
done
clear
B)
\[{{V}^{3+}}\]
done
clear
C)
\[T{{i}^{3+}}\]
done
clear
D)
\[C{{r}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 90)
Match list-I with list-II and select the correct answer using the code given below List I (successive IE) List II (Elements) \[I{{E}_{1}}\] \[I{{E}_{2}}\] \[I{{E}_{3}}\] \[(kJ\,mo{{l}^{-1}})\] 1. 1320 - - A. H 2. 520 7297 11810 B. Li 3. 900 1758 14810 C. Be 4. 800 2428 3660 D. B
A)
\[A-2,\,\,B-1,\,\,C-4,\,\,D-3\]
done
clear
B)
\[A-3,\,\,B-4,\,\,C-2,\,\,D-1\]
done
clear
C)
\[A-4,\,\,B-3,\,\,C-1,\,\,D-2\]
done
clear
D)
\[A-1,\,\,B-2,\,\,C-3,\,\,D-4\]
done
clear
View Answer play_arrow
question_answer 91) The chemical formula of feldspar is
A)
\[KAlS{{i}_{3}}{{O}_{8}}\]
done
clear
B)
\[N{{a}_{3}}Al{{F}_{6}}\]
done
clear
C)
\[NaAl{{O}_{6}}\]
done
clear
D)
\[{{K}_{2}}S{{O}_{4}}\cdot A{{l}_{2}}{{(S{{O}_{4}})}_{3}}\cdot 4Al{{(OH)}_{3}}\]
done
clear
View Answer play_arrow
question_answer 92) A radioactive substance \[_{88}{{X}^{228}}\](IIA) emits\[3\alpha \]and\[3\beta \]particles to form\[Y\]. To which group of long form of the periodic table does\[Y\] belong?
A)
\[IVA\]
done
clear
B)
\[VA\]
done
clear
C)
\[VIA\]
done
clear
D)
\[VIIA\]
done
clear
View Answer play_arrow
question_answer 93) The functional groups present in salol are
A)
\[-N{{H}_{2}}\]and\[-OR\]
done
clear
B)
\[OH\]and\[-COR\]
done
clear
C)
\[-N{{H}_{2}}\]and\[-COOH\]
done
clear
D)
\[-OH\]and\[-COOR\]
done
clear
View Answer play_arrow
question_answer 94) Which of the following statements is correct?
A)
Silicon doped with boron is an \[n-\]type semiconductor
done
clear
B)
Silicon doped with arsenic is a \[p-\]type semiconductor
done
clear
C)
Metals are good conductors of electricity
done
clear
D)
Electrical conductivity of semiconductors decreases with increasing temperature
done
clear
View Answer play_arrow
question_answer 95) Hybridisation of oxygen in diethyl ether is
A)
\[sp\]
done
clear
B)
\[s{{p}^{2}}\]
done
clear
C)
\[s{{p}^{3}}\]
done
clear
D)
\[s{{p}^{3}}d\]
done
clear
View Answer play_arrow
question_answer 96) Which one of the following salts give an acidic solution in water?
A)
\[C{{H}_{3}}COONa\]
done
clear
B)
\[N{{H}_{4}}Cl\]
done
clear
C)
\[NaCl\]
done
clear
D)
\[C{{H}_{3}}COON{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 97) Which of the following is not tetrahedral?
A)
\[BF_{4}^{-}\]
done
clear
B)
\[NH_{4}^{+}\]
done
clear
C)
\[CO_{3}^{2-}\]
done
clear
D)
\[SO_{4}^{2-}\]
done
clear
View Answer play_arrow
question_answer 98) Nitrobenzene on reduction with zinc and\[N{{H}_{4}}Cl\]gives
A)
azobenzene
done
clear
B)
aniline
done
clear
C)
hydrazobenzene
done
clear
D)
\[N-\]phenyl hydroxylamine
done
clear
View Answer play_arrow
question_answer 99) Bhopal gas tragedy of 1984 was caused by
A)
carbon monoxide
done
clear
B)
phosgene
done
clear
C)
methyl cyanate
done
clear
D)
methyl isocyanate
done
clear
View Answer play_arrow
question_answer 100) Which of the following is not a peroxy acid?
A)
Perphosphoric acid
done
clear
B)
Pemitric acid
done
clear
C)
Perdisulphuric acid
done
clear
D)
Perchloric acid
done
clear
View Answer play_arrow
question_answer 101) Name given to fossil hominid of Shivalik hills in India is
A)
Ramapithecus
done
clear
B)
Australopithecus
done
clear
C)
Pithecanthropus
done
clear
D)
Neanderthalensis
done
clear
View Answer play_arrow
question_answer 102) Vagina, oesophagus, urethra contain which type of tissue?
A)
Stratified squamous epithelium
done
clear
B)
Simple squamous epithelium
done
clear
C)
Ciliated epithelium
done
clear
D)
Columnar epithelium
done
clear
View Answer play_arrow
question_answer 103) In five kingdom system of classification of RH Whittaker, how many kingdoms contain eukaryotes?
A)
Four kingdoms
done
clear
B)
One kingdom
done
clear
C)
Two kingdoms
done
clear
D)
Three kingdoms
done
clear
View Answer play_arrow
question_answer 104) Improvement of human race through hereditary qualities is called
A)
euthenics
done
clear
B)
human heredity
done
clear
C)
human demography
done
clear
D)
eugenics
done
clear
View Answer play_arrow
question_answer 105) Mammary glands are modified
A)
sweat gland
done
clear
B)
sebaceous gland
done
clear
C)
lacrymal gland
done
clear
D)
endocrine gland
done
clear
View Answer play_arrow
question_answer 106) Inheritance of a acquired characters comes under
A)
Lamarckism
done
clear
B)
Darwinism
done
clear
C)
Neo-Lamarckism
done
clear
D)
Neo-Darwinism
done
clear
View Answer play_arrow
question_answer 107) The Mesozoic era is also called as
A)
the golden age of the amphibians
done
clear
B)
the golden age of the reptiles
done
clear
C)
the golden age of the mammals
done
clear
D)
the golden age of the birds
done
clear
View Answer play_arrow
question_answer 108) Loop of Henie is associated with
A)
excretory system
done
clear
B)
respiratory system
done
clear
C)
reproductive system
done
clear
D)
digestive system
done
clear
View Answer play_arrow
question_answer 109) Which of the following is an exclusive character of class-Mammalia?
A)
Homoiothermy
done
clear
B)
Internal fertilization
done
clear
C)
Presence of a 4-chambered heart
done
clear
D)
Presence of a muscular diaphragm
done
clear
View Answer play_arrow
question_answer 110) Symmetry in Cnidaria is
A)
radial
done
clear
B)
bilateral
done
clear
C)
pentamerous
done
clear
D)
spherical
done
clear
View Answer play_arrow
question_answer 111) Blood of earthworm is
A)
red in colour, due to dissolved haemoglobin in corpuscle
done
clear
B)
red in colour, due to dissolved haemoglobin in plasma
done
clear
C)
blue in colour, due to dissolved haemocyanin in plasma
done
clear
D)
blue in colour, due to dissolved haemocyanin in corpuscles
done
clear
View Answer play_arrow
question_answer 112) Agranulocytes are
A)
lymphocytes and monocytes
done
clear
B)
eosinophils and basophils
done
clear
C)
lymphocytes and eosinophils
done
clear
D)
basophils and monocytes
done
clear
View Answer play_arrow
question_answer 113) Parthenogenesis is a term of
A)
sexual reproduction
done
clear
B)
asexual reproduction
done
clear
C)
budding
done
clear
D)
regeneration
done
clear
View Answer play_arrow
question_answer 114) Pyramid that is never inverted
A)
energy
done
clear
B)
mass
done
clear
C)
number
done
clear
D)
size
done
clear
View Answer play_arrow
question_answer 115) Two species occupying same or overlapping area are called as
A)
sympatric
done
clear
B)
allopatric
done
clear
C)
parapatric
done
clear
D)
ring species
done
clear
View Answer play_arrow
question_answer 116) CFCs are responsible for
A)
ozone layer depletion
done
clear
B)
global warming
done
clear
C)
acid rain
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 117) Amoeba differs from Entamoeba in having
A)
contractile vacuole
done
clear
B)
pseudopodia
done
clear
C)
ectoplasm
done
clear
D)
cytostome
done
clear
View Answer play_arrow
question_answer 118) A dihybrid for qualitative trait is corssed with homozygous recessive individual of its type, the phenotypic ratio is
A)
\[1:2:1\]
done
clear
B)
\[3:1\]
done
clear
C)
\[1:1:1:1\]
done
clear
D)
\[9:3:3:1\]
done
clear
View Answer play_arrow
question_answer 119) Phenomenon of industrial melanism demonstrates
A)
reproductive isolation
done
clear
B)
induced mutation
done
clear
C)
natural selection
done
clear
D)
geographical isolation
done
clear
View Answer play_arrow
question_answer 120) At menopause there is rise in urinary excretion of
A)
FSH
done
clear
B)
STH
done
clear
C)
LH
done
clear
D)
MSH
done
clear
View Answer play_arrow
question_answer 121) Zoological name of common Indian krait is
A)
Bungarus caeruleus
done
clear
B)
Ophiophagus Hannah
done
clear
C)
Viper russeli
done
clear
D)
Naja naja
done
clear
View Answer play_arrow
question_answer 122) Study of ticks and mites is
A)
Acarology
done
clear
B)
Entomology
done
clear
C)
Malacology
done
clear
D)
Carcinology
done
clear
View Answer play_arrow
question_answer 123) In acid rain,\[S{{O}_{2}}\]accounts by
A)
\[70%\]
done
clear
B)
\[100%\]
done
clear
C)
\[50%\]
done
clear
D)
\[30%\]
done
clear
View Answer play_arrow
question_answer 124) Biosphere reserve programme started in India
A)
1986
done
clear
B)
1984
done
clear
C)
1982
done
clear
D)
1988
done
clear
View Answer play_arrow
question_answer 125) Urea formation takes place in
A)
liver
done
clear
B)
kidney
done
clear
C)
pancreas
done
clear
D)
intestine
done
clear
View Answer play_arrow
question_answer 126) An institution where valuable plant material-likely to become irretrievably lost in the wild or in cultivation is preserved viable condition is known as
A)
genome
done
clear
B)
gene library
done
clear
C)
gene bank
done
clear
D)
herbarium
done
clear
View Answer play_arrow
question_answer 127) Shock movement in touch me not plant is
A)
seismonasty
done
clear
B)
photo nasty
done
clear
C)
chemonasty
done
clear
D)
the rmonasty
done
clear
View Answer play_arrow
question_answer 128) Which is correct for the structure of cell wall of bacteria and fungi?
A)
Both are made up of cellulose
done
clear
B)
Both have mucopeptide
done
clear
C)
Both are made up of N-acetylglucosamine
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 129) Bacterium which reduces nitrates in soil to nitrogen is
A)
Nitro so monas
done
clear
B)
Pseudomonas
done
clear
C)
Rhizobium
done
clear
D)
Clostridium
done
clear
View Answer play_arrow
question_answer 130) Surrounding membrane of vacoule is called
A)
tonoplast
done
clear
B)
symplast
done
clear
C)
apoplast
done
clear
D)
phragmoplast
done
clear
View Answer play_arrow
question_answer 131) Living fossil is
A)
Ginkgo biloba
done
clear
B)
Gnetum ula
done
clear
C)
Pinus roxburghii
done
clear
D)
Cycas revolute
done
clear
View Answer play_arrow
question_answer 132) Sucking roots are present in the plant
A)
Betal
done
clear
B)
Cuscuta
done
clear
C)
Mangifera
done
clear
D)
Solanum
done
clear
View Answer play_arrow
question_answer 133) For cryopreservation, plant materials are frozen at
A)
\[-{{196}^{o}}C\]
done
clear
B)
\[-{{150}^{o}}C\]
done
clear
C)
\[-{{80}^{o}}C\]
done
clear
D)
\[-{{40}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 134) Casparian strip is a characteristic feature of
A)
pericycle
done
clear
B)
periblem
done
clear
C)
endodermis
done
clear
D)
hypodermis
done
clear
View Answer play_arrow
question_answer 135) Guttation occurs through
A)
lenticels
done
clear
B)
hydathodes
done
clear
C)
periderm
done
clear
D)
stomata
done
clear
View Answer play_arrow
question_answer 136) Phycology is the study of
A)
algae
done
clear
B)
fern
done
clear
C)
fungi
done
clear
D)
bryophytes
done
clear
View Answer play_arrow
question_answer 137) ......... is a CAM plant.
A)
maize
done
clear
B)
pineapple
done
clear
C)
onion
done
clear
D)
pea
done
clear
View Answer play_arrow
question_answer 138) Edible part of the apple is
A)
mesocarp
done
clear
B)
calyx
done
clear
C)
thalamus
done
clear
D)
pericarp
done
clear
View Answer play_arrow
question_answer 139) Sunken stomata is found in leaves of
A)
Trifolium
done
clear
B)
Lemna
done
clear
C)
Nerium
done
clear
D)
Lilium
done
clear
View Answer play_arrow
question_answer 140) The accumulated food reserve in fungi is
A)
protein
done
clear
B)
starch
done
clear
C)
glycogen
done
clear
D)
fat
done
clear
View Answer play_arrow
question_answer 141) Euploidy is best explained by
A)
exact multiple of a haploid set of chromosomes
done
clear
B)
one chromosomes less than the haploid set of chromosomes
done
clear
C)
one chromosomes more than the haploid set of chromosomes
done
clear
D)
one chromosomes more than the diploid set of chromosomes
done
clear
View Answer play_arrow
question_answer 142) Insectivorous plants live in a soil that is usually deficient in
A)
nitrate
done
clear
B)
chloride
done
clear
C)
potassium
done
clear
D)
magnesium
done
clear
View Answer play_arrow
question_answer 143) In aerobic respiration, citric acid cycle takes place in
A)
cytosol
done
clear
B)
mitochondria
done
clear
C)
peroxisomes
done
clear
D)
endoplasmic reticulum
done
clear
View Answer play_arrow
question_answer 144) The highest advanced family is
A)
Cruciferae
done
clear
B)
Cucurbitaceae
done
clear
C)
Compositae
done
clear
D)
Euphorbiaceae
done
clear
View Answer play_arrow
question_answer 145) Stalk with which ovules remain attached to the placenta is called
A)
funicle
done
clear
B)
raphe
done
clear
C)
hilum
done
clear
D)
chalaza
done
clear
View Answer play_arrow
question_answer 146) In pteridophytes, phloem is without
A)
sieve cells
done
clear
B)
sieve tubes
done
clear
C)
companion cells
done
clear
D)
bast fibres
done
clear
View Answer play_arrow
question_answer 147) Purified antibiotic penicillin of Penicillium notatum was discovered by
A)
Alexander Flemming
done
clear
B)
Howard Floxy
done
clear
C)
Robert Hooke
done
clear
D)
Carolus Linnaeus
done
clear
View Answer play_arrow
question_answer 148) First vascular plant is
A)
Thallophyta
done
clear
B)
Bryophyta
done
clear
C)
Pteridophyta
done
clear
D)
Spermatophyta
done
clear
View Answer play_arrow
question_answer 149) Bakers yeast is
A)
S. cerevisae
done
clear
B)
S. ludwingii
done
clear
C)
S. octosporus
done
clear
D)
Schizosdccharomyces
done
clear
View Answer play_arrow
question_answer 150) Dominant generation in bryophytes is
A)
capsule
done
clear
B)
sporophyte
done
clear
C)
gametophyte
done
clear
D)
seta
done
clear
View Answer play_arrow
question_answer 151) Largest flower is
A)
Rafflesia arnnoldi
done
clear
B)
Helianthus annuus
done
clear
C)
Welwitschia morabilis
done
clear
D)
Nelumbo nuccifera
done
clear
View Answer play_arrow
question_answer 152) Halophytes are
A)
fire resistant
done
clear
B)
cold resistant
done
clear
C)
salt resistant
done
clear
D)
sand loving
done
clear
View Answer play_arrow
question_answer 153) Natural genetic engineer is
A)
Bacillus subtilis
done
clear
B)
Pseudomonas sp.
done
clear
C)
Escherichia coli
done
clear
D)
Agrobacterium tumefaciens
done
clear
View Answer play_arrow
question_answer 154) Cell respiration is carried out by
A)
ribosome
done
clear
B)
mitochondria
done
clear
C)
chloroplast
done
clear
D)
Golgi bodies
done
clear
View Answer play_arrow
question_answer 155) Fluid mosaic model of plasma membrane was given by
A)
Robertson
done
clear
B)
Robert Hooke
done
clear
C)
Singer and Nicholson
done
clear
D)
Pantin and Mast
done
clear
View Answer play_arrow
question_answer 156) Which amino acids are present in histones?
A)
Lysine and histidine
done
clear
B)
Valine and histidine
done
clear
C)
Arginine and lysine
done
clear
D)
Arginine and histidine
done
clear
View Answer play_arrow
question_answer 157) Which has vascular tissue, produces spores, but does not has seeds?
A)
Bryophyta
done
clear
B)
Pteridophyta
done
clear
C)
Gymnosperms
done
clear
D)
Angiosperms
done
clear
View Answer play_arrow
question_answer 158) Which enzyme joins DNA fragments?
A)
DNA ligase
done
clear
B)
DNA polymerase
done
clear
C)
DNA gyrase
done
clear
D)
Topoisomerase
done
clear
View Answer play_arrow
question_answer 159) The structure present in cyanobacteria (BGA) helping in\[{{N}_{2}}\]fixation is
A)
haplosperm
done
clear
B)
holostrum
done
clear
C)
holotrema
done
clear
D)
heterocyst
done
clear
View Answer play_arrow
question_answer 160) RNA has uracil instead of
A)
cytosine
done
clear
B)
guanine
done
clear
C)
thymine
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 161) Endosperm of gymnosperm is
A)
diploid
done
clear
B)
tetraploid
done
clear
C)
haploid
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 162) Binomial nomenclature of plants was given by
A)
Engler
done
clear
B)
Linnaeus
done
clear
C)
Prand
done
clear
D)
Both [a] and [c]
done
clear
View Answer play_arrow
question_answer 163) Bacterial blight of rice is caused due to
A)
Xanthomonas oryzae
done
clear
B)
Helminthosporium oryzae
done
clear
C)
Pseudomonas falcatum
done
clear
D)
Xanthomonas falcatum
done
clear
View Answer play_arrow
question_answer 164) Female reproductive part of bryophytes is
A)
antheridium
done
clear
B)
oogonium
done
clear
C)
archegonium
done
clear
D)
sporangium
done
clear
View Answer play_arrow
question_answer 165) Chitin is present in the cell wall of
A)
fungi
done
clear
B)
bacteria
done
clear
C)
yeast
done
clear
D)
algae
done
clear
View Answer play_arrow
question_answer 166) Triple fusion in angiosperm is the fusion of second sperm with
A)
antipodal cell and one synergid cell
done
clear
B)
two antipodal cells
done
clear
C)
two synergid cells
done
clear
D)
two polar nuclei
done
clear
View Answer play_arrow
question_answer 167) Father of botany is
A)
Linnaeus
done
clear
B)
Leeuwenhoek
done
clear
C)
Theophrastus
done
clear
D)
Aristotle
done
clear
View Answer play_arrow
question_answer 168) Hormone inducing fruit ripening is
A)
ethylene
done
clear
B)
cytokinin
done
clear
C)
gibberellic acid
done
clear
D)
abscisic acid
done
clear
View Answer play_arrow
question_answer 169) Red colour of tomato is due to
A)
\[\beta -\]carotene
done
clear
B)
anthocyanin
done
clear
C)
lycopene
done
clear
D)
erythrocyanin
done
clear
View Answer play_arrow
question_answer 170) In which phase proteins for spindle fibre formation are synthesized?
A)
\[{{G}_{1}}-\]phase
done
clear
B)
\[{{G}_{2}}-\]phase
done
clear
C)
\[S-\]phase
done
clear
D)
Anaphase
done
clear
View Answer play_arrow
question_answer 171) Desert grasses often roll their leaves due to presence of
A)
oily surface
done
clear
B)
bulli form cells
done
clear
C)
spines
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 172) \[{{F}_{1}}\]particles are present in
A)
chloroplast
done
clear
B)
mitochondria
done
clear
C)
ribosome
done
clear
D)
rough ER
done
clear
View Answer play_arrow
question_answer 173) Sucrose, a common table sugar is composed of
A)
glucose + fructose
done
clear
B)
glucose + galactose
done
clear
C)
fructose + galactose
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 174) Which is not true about sphaerosomes?
A)
Arise from ER
done
clear
B)
Related to fat
done
clear
C)
Single membrane bound structure
done
clear
D)
Involved in photorespiration
done
clear
View Answer play_arrow
question_answer 175) The species listed in Red Data book are
A)
threatened
done
clear
B)
endangered
done
clear
C)
rare
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 176) Inflorescence of family-Compositae is
A)
perianth
done
clear
B)
iodicule
done
clear
C)
capitulum
done
clear
D)
hypanthodium
done
clear
View Answer play_arrow
question_answer 177) Prokaryotic ribosome has sedimentation coefficient of
A)
\[80S\]
done
clear
B)
\[70S\]
done
clear
C)
\[40S\]
done
clear
D)
\[60S\]
done
clear
View Answer play_arrow
question_answer 178) The condition where filaments and anthers are fused throughout the entire length is
A)
synandrous
done
clear
B)
gynandrous
done
clear
C)
protandrous
done
clear
D)
syngenesius
done
clear
View Answer play_arrow
question_answer 179) In diot stem, vascular bundles are
A)
numerous scattered
done
clear
B)
arranged in a ring
done
clear
C)
without cambium
done
clear
D)
surrounded by bundle sheath
done
clear
View Answer play_arrow
question_answer 180) Neurons of people suffering from diabetes insipidus do not secrete
A)
enzyme
done
clear
B)
steroid
done
clear
C)
fattyacid
done
clear
D)
ADH
done
clear
View Answer play_arrow
question_answer 181) Insulin and glucagon are transported to target organ by
A)
lymph
done
clear
B)
blood
done
clear
C)
pancreatic duct
done
clear
D)
cystic duct
done
clear
View Answer play_arrow
question_answer 182) Which is false for nutrition in Amoeba?
A)
Omnivorous
done
clear
B)
Pseudopodia feeder
done
clear
C)
Holozoic nutrition
done
clear
D)
Photoautotroph
done
clear
View Answer play_arrow
question_answer 183) Ions that must be present for binding the cross bridges is
A)
\[N{{a}^{+}}\]
done
clear
B)
\[C{{a}^{2+}}\]
done
clear
C)
\[{{K}^{+}}\]
done
clear
D)
\[M{{g}^{+}}\]
done
clear
View Answer play_arrow
question_answer 184) Dreaming occurs in
A)
\[\alpha -\]sleep
done
clear
B)
REM sleep
done
clear
C)
deep sleep
done
clear
D)
slow wave sleep
done
clear
View Answer play_arrow
question_answer 185) Study of behaviour of animal is called
A)
ethology
done
clear
B)
parapsychology
done
clear
C)
euphenics
done
clear
D)
etiology
done
clear
View Answer play_arrow
question_answer 186) Minamata disease is caused due to presence of ......... in water.
A)
cadmium
done
clear
B)
lead
done
clear
C)
arsenic
done
clear
D)
mercury
done
clear
View Answer play_arrow
question_answer 187) Rain is called acid rain when its pH is below
A)
7
done
clear
B)
6.5
done
clear
C)
6
done
clear
D)
5.6
done
clear
View Answer play_arrow
question_answer 188) The immediate cause of induction of ovulation in female is the large plasma surge of
A)
progesterone
done
clear
B)
estriadiol
done
clear
C)
LH
done
clear
D)
FSH
done
clear
View Answer play_arrow
question_answer 189) HIV virus affect......... in AIDS patient.
A)
cytotoxic T-cell
done
clear
B)
M-N cell
done
clear
C)
suppressor cell
done
clear
D)
helper T-cells
done
clear
View Answer play_arrow
question_answer 190) Which is incorrect?
A)
Wings of insects and birds are analogous
done
clear
B)
Wings of insects and bats are analogous
done
clear
C)
Wings of insects and birds are homologous
done
clear
D)
Wings of bats and birds are homologous
done
clear
View Answer play_arrow
question_answer 191) Dental formula of human being is
A)
\[{{I}_{2}},\,\,{{C}_{2}},\,\,{{P}_{1}},\,\,{{M}_{3}}\]
done
clear
B)
\[{{I}_{2}},\,\,{{C}_{1}},\,\,{{P}_{2}},\,\,{{M}_{3}}\]
done
clear
C)
\[{{I}_{3}},\,\,{{C}_{1}},\,\,{{P}_{2}},\,\,{{M}_{2}}\]
done
clear
D)
\[{{I}_{2}},\,\,{{C}_{2}},\,\,{{P}_{3}},\,\,{{M}_{1}}\]
done
clear
View Answer play_arrow
question_answer 192) Which is not correctly matched?
A)
Annedlida\[\to \]Enterocoelomate
done
clear
B)
Platyhelminthes\[\to \]Acoelomate
done
clear
C)
Arthropoda\[\to \]Schizocoelomate
done
clear
D)
Nemathelminthes\[\to \]Pseudocoelomate
done
clear
View Answer play_arrow
question_answer 193) Maximum net productivity in the terrestrial ecosystem is in
A)
rain forest
done
clear
B)
deciduous forest
done
clear
C)
mangrove plantation
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 194) ......... acts as a shock absorber to cushion when tibia and femur came together.
A)
ligament
done
clear
B)
cartilage
done
clear
C)
tendon
done
clear
D)
disc
done
clear
View Answer play_arrow
question_answer 195) In higher vertebrates, SA nodes helps in
A)
conduction of blood
done
clear
B)
initiation of heart beat
done
clear
C)
opening of tricuspid valve
done
clear
D)
opening of bicuspid valve
done
clear
View Answer play_arrow
question_answer 196) Renin is secreted from
A)
juxtaglomerular cells
done
clear
B)
podocytes
done
clear
C)
nephridia
done
clear
D)
stomach
done
clear
View Answer play_arrow
question_answer 197) The law of limiting factors was proposed with particular reference to photosynthesis. Identify the scientist who proposed this law?
A)
Calvin
done
clear
B)
Weismann
done
clear
C)
Emerson
done
clear
D)
Blackmann
done
clear
View Answer play_arrow
question_answer 198) Osmoregulation in Paramecium is a function of
A)
contractile vacuole
done
clear
B)
trichocysts
done
clear
C)
cytopyge
done
clear
D)
cytostome
done
clear
View Answer play_arrow
question_answer 199) Which of the following is a day neutral plant?
A)
Helianthus annuus
done
clear
B)
Euphorbia pulcherrima
done
clear
C)
Avena sativa
done
clear
D)
Betavulgaris
done
clear
View Answer play_arrow
question_answer 200) Water vascular system found in
A)
Mollusca
done
clear
B)
Arthropoda
done
clear
C)
Annelida
done
clear
D)
Echinodermata
done
clear
View Answer play_arrow