question_answer 1) A cane filled with water is revolved in a vertical circle of radius 4 m and the water does not fall down. What must be the maximum period of revolution?
A)
\[2s\]
done
clear
B)
\[4s\]
done
clear
C)
\[1s\]
done
clear
D)
\[6s\]
done
clear
View Answer play_arrow
question_answer 2) The radius R of the soap bubble is doubled under isothermal condition. If\[T\]be the surface tension of soap bubble, the required surface energy in doing so is given by
A)
\[23\pi {{R}^{2}}T\]
done
clear
B)
\[24\pi {{R}^{2}}T\]
done
clear
C)
\[8\pi {{R}^{2}}T\]
done
clear
D)
\[4\pi {{R}^{2}}T\]
done
clear
View Answer play_arrow
question_answer 3) To make the frequency double of a spring oscillator, we have to
A)
reduce the mass to one-fourth
done
clear
B)
quadruple the mass
done
clear
C)
double the mass
done
clear
D)
half the mass
done
clear
View Answer play_arrow
question_answer 4) A short linear object of length b lies along the axis of a concave mirror of focal length \[f\] at a distance u from the pole of the mirror, what is the size of image?
A)
\[\left( \frac{f}{u-f} \right)b\]
done
clear
B)
\[{{\left( \frac{f}{u-f} \right)}^{2}}b\]
done
clear
C)
\[\left( \frac{f}{u-f} \right){{b}^{2}}\]
done
clear
D)
\[\left( \frac{f}{u-f} \right)\]
done
clear
View Answer play_arrow
question_answer 5) The difference between the apparent frequency of a source of sound as perceived by the observer during its approach and recession is \[2%\] of the frequency of the source. If the speed of sound in air is \[300\text{ }m{{s}^{-1}}\], the velocity of the source is
A)
\[1.5m{{s}^{-1}}\]
done
clear
B)
\[12m{{s}^{-1}}\]
done
clear
C)
\[6m{{s}^{-1}}\]
done
clear
D)
\[3m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 6) A count rate metre shows a count of 240/min from a given radioactive source later the metre shows a count rate of 30/min. The half-life of the source is
A)
80min
done
clear
B)
120 min
done
clear
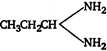
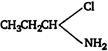
C)
20 min
done
clear
D)
30 min
done
clear
View Answer play_arrow
question_answer 7) Curie-Weiss law is obeyed by iron
A)
at Curie temperature only
done
clear
B)
at all temperatures
done
clear
C)
below Curie temperature
done
clear
D)
above Curie temperature
done
clear
View Answer play_arrow
question_answer 8) The energy of an electron in an excited hydrogen atom is\[-3.4eV\]. Its angular momentum is
A)
\[3.72\times {{10}^{-34}}J-s\]
done
clear
B)
\[2.11\times {{10}^{-34}}J-s\]
done
clear
C)
\[1.57\times {{10}^{-34}}J-s\]
done
clear
D)
\[1.11\times {{10}^{-34}}J-s\]
done
clear
View Answer play_arrow
question_answer 9) A tuning fork of frequency \[340Hz\] is vibrated just above the tube of \[120cm\] height. Water is poured slowly in the tube. What is the minimum height of water necessary for the resonance? (speed of sound in air\[=340m/s\])
A)
\[45cm\]
done
clear
B)
\[30cm\]
done
clear
C)
\[40cm\]
done
clear
D)
\[25cm\]
done
clear
View Answer play_arrow
question_answer 10) The escape velocity of a projectile on the earths surface is\[11.2km{{s}^{-1}}\]. A body is projected out with thrice this speed. The speed of the body far away from the earth will be
A)
\[22.4km\,\,{{s}^{-1}}\]
done
clear
B)
\[31.7km\,\,{{s}^{-1}}\]
done
clear
C)
\[33.6km\,\,{{s}^{-1}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 11) The range of particle when launched at an angle of \[{{15}^{o}}\] with the horizontal is\[1.5km\]. What is the range of the projectile when launched at an angle of \[{{45}^{o}}\] to the horizontal?
A)
\[3.0km\]
done
clear
B)
\[1.5km\]
done
clear
C)
\[6.0km\]
done
clear
D)
\[0.75km\]
done
clear
View Answer play_arrow
question_answer 12) A gas is compressed at a constant pressure of \[50N/{{m}^{2}}\] from a volume of \[10{{m}^{3}}\] to a volume of\[4{{m}^{3}}\]. Energy of \[100J\] is thus added to die gas by heating. Its internal energy is
A)
decreased by\[200J\]
done
clear
B)
increased by\[100J\]
done
clear
C)
increased by\[300J\]
done
clear
D)
increased by\[400J\]
done
clear
View Answer play_arrow
question_answer 13)
A square wire of each side I carries a current J. What is the magnetic field at the midpoint of the square?
A)
\[4\sqrt{2}\frac{{{\mu }_{0}}}{4\pi }\frac{I}{l}\]
done
clear
B)
\[8\sqrt{2}\frac{{{\mu }_{0}}}{4\pi }\frac{I}{l}\]
done
clear
C)
\[16\sqrt{2}\frac{{{\mu }_{0}}}{4\pi }\frac{I}{l}\]
done
clear
D)
\[32\sqrt{2}\frac{{{\mu }_{0}}}{4\pi }\frac{I}{l}\]
done
clear
View Answer play_arrow
question_answer 14) When a wire is stretched and its radius becomes\[r/2\], then its resistance will be
A)
\[16R\]
done
clear
B)
\[4R\]
done
clear
C)
\[2R\]
done
clear
D)
\[zero\]
done
clear
View Answer play_arrow
question_answer 15) A cell of emf\[X\]is connected across a resistor R. The potential difference across the wire is measured as V. The internal resistance of the cell should be
A)
\[X\text{--}Y/R\]
done
clear
B)
\[(X-Y)R/Y\]
done
clear
C)
\[(X-Y)Y/R\]
done
clear
D)
\[(X-Y)R\]
done
clear
View Answer play_arrow
question_answer 16) Light of wavelength \[0.6\mu m\] from a sodium lamp falls on a photocell and causes the emission of photoelectrons for which the stopping potential is \[0.5V\] with light of wavelength \[0.4\mu m\] from a sodium lamp, the stopping potential is \[1.5V\], with this data, the value of\[h/e\]is
A)
\[4\times {{10}^{-19}}V\text{-}s\]
done
clear
B)
\[0.25\times {{10}^{-15}}V\text{-}s\]
done
clear
C)
\[4\times {{10}^{-15}}V\text{-}s\]
done
clear
D)
\[4\times {{10}^{-8}}V\text{-}s\]
done
clear
View Answer play_arrow
question_answer 17)
In the inductive circuit given in the figure, the currents rises after the switch is closed. At instant when the current is \[15mA\], then potential difference across the inductor will be
A)
zero
done
clear
B)
240 V
done
clear
C)
180 V
done
clear
D)
60 V
done
clear
View Answer play_arrow
question_answer 18) If the power factor changes from\[\frac{1}{2}\]to\[\frac{1}{4}\], then what is the increase in impedance in\[AC\]?
A)
\[20%\]
done
clear
B)
\[50%\]
done
clear
C)
\[25%\]
done
clear
D)
\[100%\]
done
clear
View Answer play_arrow
question_answer 19) The earth absorbs \[1022J\] of energy from the sum every day. The percentage increase in the mass of earth every day will be (mass of earth \[=6\times {{10}^{24}}kg\])
A)
\[1.82\times {{10}^{-10}}%\]
done
clear
B)
\[1.85\times {{10}^{-15}}%\]
done
clear
C)
\[1.85\times {{10}^{-17}}%\]
done
clear
D)
\[1.85\times {{10}^{-18}}%\]
done
clear
View Answer play_arrow
question_answer 20) Every series of hydrogen spectrum has an upper and lower limit in wavelength. The spectral series which has an upper limit of wavelength equal to\[18752\overset{\text{o}}{\mathop{\text{A}}}\,\], is
A)
Balmer series
done
clear
B)
Lyman series
done
clear
C)
Paschan series
done
clear
D)
Pound series
done
clear
View Answer play_arrow
question_answer 21) A body of mass m moving with velocity \[v\] makes a head-on collision with another body of mass \[2m\] which is initially at rest. The loss of kinetic energy of the colliding body (mass\[m\]) is
A)
\[\frac{1}{2}\]of its initial kinetic energy
done
clear
B)
\[\frac{1}{9}\]of its initial kinetic energy
done
clear
C)
\[\frac{8}{9}\]of its initial kinetic energy
done
clear
D)
\[\frac{1}{4}\]of its initial kinetic energy
done
clear
View Answer play_arrow
question_answer 22) An ice cube of density \[900kg/{{m}^{3}}\] floating in water of density\[1000kg/{{m}^{3}}\]. The percentage of volume of ice-cube outside the water is
A)
\[20%\]
done
clear
B)
\[35%\]
done
clear
C)
\[10%\]
done
clear
D)
\[25%\]
done
clear
View Answer play_arrow
question_answer 23) A voltmeter having a resistance of \[998\Omega \] is connected to a cell of emf \[2V\] and internal resistance\[2\Omega \]. The error in the measurement of emf will be
A)
\[4\times {{10}^{-1}}V\]
done
clear
B)
\[2\times {{10}^{-3}}V\]
done
clear
C)
\[4\times {{10}^{-3}}V\]
done
clear
D)
\[2\times {{10}^{-1}}V\]
done
clear
View Answer play_arrow
question_answer 24) The deceleration experienced by a moving motor boat, after its engine is cut-off is given\[\frac{dv}{dt}=-k{{v}^{3}}\]where \[k\] is constant and \[{{v}_{0}}\] is the magnitude of the velocity at time \[t\] after the cut off is
A)
\[{{v}_{0}}{{c}^{-kt}}\]
done
clear
B)
\[\frac{{{v}_{0}}}{\sqrt{2v_{0}^{2}kt+1}}\]
done
clear
C)
\[{{v}_{0}}\]
done
clear
D)
\[\frac{{{v}_{0}}}{2}\]
done
clear
View Answer play_arrow
question_answer 25) The dimensions of\[\frac{{{e}^{2}}}{4\pi {{\varepsilon }_{0}}hc}\], where\[e,\,{{\varepsilon }_{0}},\,h\]and\[c\] are electronic charge, electric permittivity Plancks constant and velocity of light in vacuum respectively
A)
\[[{{M}^{0}}{{L}^{0}}{{T}^{0}}]\]
done
clear
B)
\[[M{{L}^{0}}{{T}^{0}}]\]
done
clear
C)
\[[{{M}^{0}}L{{T}^{0}}]\]
done
clear
D)
\[[{{M}^{0}}{{L}^{0}}T]\]
done
clear
View Answer play_arrow
question_answer 26) A stone is dropped from a height \[h\] simultaneously, another stone is thrown up from the ground which reaches a height\[4h\]. The two stones cross each other after time
A)
\[\sqrt{\frac{h}{8g}}\]
done
clear
B)
\[\sqrt{8gh}\]
done
clear
C)
\[\sqrt{2gh}\]
done
clear
D)
\[\sqrt{\left( \frac{h}{2g} \right)}\]
done
clear
View Answer play_arrow
question_answer 27) A spring balance and a physical balance are kept in a lift. In these balances equal masses are placed. If now the lift starts moving upwards with constant acceleration, then
A)
the reading of spring balance will increase and the equilibrium position of the physical balance will disturb
done
clear
B)
the reading of spring balance will remain unchanged and physical balance will remain in equilibrium
done
clear
C)
the reading of spring balance will decrease and physical balance will remain in equilibrium
done
clear
D)
the reading of spring balance will increase and the physical balance will remain in equilibrium
done
clear
View Answer play_arrow
question_answer 28)
A rough vertical board has an acceleration \[d\] so that a \[2kg\] block pressing against it does not fall. The coefficient of friction between the block and the board should be
A)
\[>g/a\]
done
clear
B)
\[<g/a\]
done
clear
C)
\[=g/a\]
done
clear
D)
\[>a/g\]
done
clear
View Answer play_arrow
question_answer 29) A reel of thread unrolls itself falling under gravity. Neglecting mass of the thread, the acceleration of the reel is
A)
\[g\]
done
clear
B)
\[\frac{g}{2}\]
done
clear
C)
\[\frac{2g}{3}\]
done
clear
D)
\[\frac{4g}{3}\]
done
clear
View Answer play_arrow
question_answer 30) A particle is moving in a vertical circle. The tensions in the string when passing through two positions at angle \[{{30}^{o}}\] and \[{{60}^{o}}\] from vertical (lowest position) are \[{{T}_{1}}\] and \[{{T}_{2}}\]respectively, then
A)
\[{{T}_{1}}={{T}_{2}}\]
done
clear
B)
\[{{T}_{2}}>{{T}_{1}}\]
done
clear
C)
\[{{T}_{1}}>{{T}_{2}}\]
done
clear
D)
tension in the string always remains the same
done
clear
View Answer play_arrow
question_answer 31) When an ideal diatomic gas is heated at constant pressure, the fraction of the heat energy supplied which increases, the internal energy of the gas, is
A)
\[\frac{2}{5}\]
done
clear
B)
\[\frac{3}{5}\]
done
clear
C)
\[\frac{3}{7}\]
done
clear
D)
\[\frac{5}{7}\]
done
clear
View Answer play_arrow
question_answer 32) A black body radiates \[20W\] temperature\[{{227}^{o}}C\]. If temperature of the black body is changed to \[{{727}^{o}}C\] then its radiating power will be
A)
\[120W\]
done
clear
B)
\[240W\]
done
clear
C)
\[320W\]
done
clear
D)
\[360W\]
done
clear
View Answer play_arrow
question_answer 33) A stretched wire of length \[110cm\] is divided into three segments whose frequencies are in ratio\[1:2:3\]. Their length must be
A)
20 cm; 30 cm; 60 cm
done
clear
B)
60 cm; 30 cm; 20 cm
done
clear
C)
60 cm; 20 cm; 30 cm
done
clear
D)
30 cm; 60 cm; 20 cm
done
clear
View Answer play_arrow
question_answer 34) The power of a sound from the speaker of a radio is\[20mW\]. By turning the knob of the volume control, the power of the sound is increased to\[400mW\]. The power increase in decibels as compared to the original power is
A)
\[13dB\]
done
clear
B)
\[10dB\]
done
clear
C)
\[20dB\]
done
clear
D)
\[800dB\]
done
clear
View Answer play_arrow
question_answer 35)
Two identical capacitors A and B are charged to the same potential V and are connected in two circuits ate = 0, as shown in figure. The charge on the capacitors at timer = CR are respectively
A)
\[VC,\,VC\]
done
clear
B)
\[\frac{VC}{e},\,\,VC\]
done
clear
C)
\[VC,\,\,\frac{VC}{e}\]
done
clear
D)
\[\frac{VC}{e},\,\,\frac{VC}{e}\]
done
clear
View Answer play_arrow
question_answer 36) In the following common emitter circuit if\[\beta =100\],\[{{V}_{CE}}=7V\],\[{{V}_{BE}}=\]Neglegible,\[{{R}_{C}}=2k\Omega \]then
A)
\[0.01mA\]
done
clear
B)
\[0.04mA\]
done
clear
C)
\[0.02mA\]
done
clear
D)
\[0.03mA\]
done
clear
View Answer play_arrow
question_answer 37)
The following configuration of gate is equivalent to
A)
\[NAND\]
done
clear
B)
\[XOR\]
done
clear
C)
\[OR\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 38) In an electromagnetic wave, the electric and magnetising fields are \[100V{{m}^{-1}}\] and\[0.265A{{m}^{-1}}\]. The maximum energy flow is
A)
\[26.5\,\,W/{{m}^{2}}\]
done
clear
B)
\[36.5\,\,W/{{m}^{2}}\]
done
clear
C)
\[46.7\,\,W/{{m}^{2}}\]
done
clear
D)
\[765\,\,W/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 39) A copper wire of length 1m and radius \[1mm\] is joined in series with an iron wire of length \[2m\] and radius \[3mm\] and a current is passed through the wires. The ratio of the current density in the copper and iron wires is
A)
\[18:1\]
done
clear
B)
\[9:1\]
done
clear
C)
\[6:1\]
done
clear
D)
\[2:3\]
done
clear
View Answer play_arrow
question_answer 40) If the momentum of an electron is changed by\[{{\Delta }_{p}}\], then the de-Broglie wavelength associated with it changes by\[0.50%\]. The initial momentum of the electron will be
A)
\[\frac{\Delta p}{200}\]
done
clear
B)
\[\frac{\Delta p}{199}\]
done
clear
C)
\[199\,\,\Delta p\]
done
clear
D)
\[400\,\,\Delta p\]
done
clear
View Answer play_arrow
question_answer 41) An object is placed at a distance of\[\frac{f}{2}\]from a convex lens. The image will be
A)
at one of the foci, virtual and double its size
done
clear
B)
at\[\frac{3f}{2}\], real and inverted
done
clear
C)
at\[2f\], virtual and erect
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 42) The maximum intensity of fringes in Youngs experiment is J. If one of the slit is closed then the intensity at that place becomes\[{{I}_{0}}\]. Which of the following relation is true?
A)
\[I={{I}_{0}}\]
done
clear
B)
\[I=2{{I}_{0}}\]
done
clear
C)
\[I=4{{I}_{0}}\]
done
clear
D)
There is no relation between\[I\]and\[{{I}_{0}}\]
done
clear
View Answer play_arrow
question_answer 43) A simple telescope consisting of an objective of focal length \[60cm\] and a single eye lens of focal length \[5cm\] is focussed on a distant object in such a way that parallel rays comes out from the eye lens. If the object subtends an angle \[{{2}^{o}}\] at the objective, the angular width of the image is
A)
\[10{}^\circ \]
done
clear
B)
\[24{}^\circ \]
done
clear
C)
\[50{}^\circ \]
done
clear
D)
\[\frac{1}{6}{}^\circ \]
done
clear
View Answer play_arrow
question_answer 44)
In the following figure two parallel metallic plates are maintained at different potential. If an electron is released midway between the plates, it will move
A)
right ward at constant speed
done
clear
B)
left ward at constant speed
done
clear
C)
accelerated right ward
done
clear
D)
accelerated left ward
done
clear
View Answer play_arrow
question_answer 45) Charge \[q\] is uniformly distributed over a thin half ring of radius\[R\]. The electric field at the centre of the ring is
A)
\[\frac{q}{2{{\pi }^{2}}{{\varepsilon }_{0}}{{R}^{2}}}\]
done
clear
B)
\[\frac{q}{4{{\pi }^{2}}{{\varepsilon }_{0}}{{R}^{2}}}\]
done
clear
C)
\[\frac{q}{4\pi {{\varepsilon }_{0}}{{R}^{2}}}\]
done
clear
D)
\[\frac{q}{2\pi {{\varepsilon }_{0}}{{R}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 46)
Consider a parallel plate capacitor of \[10\mu F\] (micro-farad) with air filled in the gap between the plates. Now one-half of the space between the plates is filled with a dielectric of dielectric constant 4, as shown in the figure. The capacity of the capacitor changes to
A)
\[25\mu F\]
done
clear
B)
\[20\mu F\]
done
clear
C)
\[40\mu F\]
done
clear
D)
\[5\mu F\]
done
clear
View Answer play_arrow
question_answer 47) If\[\mathbf{\vec{A}}=2\mathbf{\hat{i}}+3\mathbf{\hat{j}}-\mathbf{\hat{k}}\]and\[\mathbf{\vec{B}}=-\mathbf{\hat{i}}+3\mathbf{\hat{j}}+4\mathbf{\hat{k}}\], then projection of\[\mathbf{\vec{A}}\]on\[\mathbf{\vec{B}}\]will be
A)
\[\frac{3}{\sqrt{13}}\]
done
clear
B)
\[\frac{3}{\sqrt{26}}\]
done
clear
C)
\[\sqrt{\frac{3}{26}}\]
done
clear
D)
\[\sqrt{\frac{3}{13}}\]
done
clear
View Answer play_arrow
question_answer 48)
A string of length \[L\] is fixed at one end and carries a mass \[M\] at the other end. The string makes \[\frac{2}{\pi }\] revolutions per second around the vertical axis through the fixed end as shown in the figure, then tension in the string is
A)
\[ML\]
done
clear
B)
\[2ML\]
done
clear
C)
\[4ML\]
done
clear
D)
\[16ML\]
done
clear
View Answer play_arrow
question_answer 49) Two identical induction coils each of inductance \[L\] joined in series are placed very close to each other such that the winding direction of one is exactly opposite to that of the other, what is net inductance?
A)
\[{{L}^{2}}\]
done
clear
B)
\[2L\]
done
clear
C)
\[\frac{L}{2}\]
done
clear
D)
\[Zero\]
done
clear
View Answer play_arrow
question_answer 50) A beaker is completely filled with water at\[{{4}^{o}}C.\] It will overflow if
A)
heated above\[{{4}^{o}}C\]
done
clear
B)
cooled below\[{{4}^{o}}C\]
done
clear
C)
both heated and cooled above and below\[{{4}^{o}}C\]respectively
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 51) \[_{92}{{U}^{235}}\] nucleus absorb a neutron and disintegrate in\[_{54}X{{e}^{139}},\,{{\,}_{38}}S{{r}^{94}}\]and\[X\] so, what will be product\[X\]
A)
\[3-\]neutrons
done
clear
B)
\[2-\]neutrons
done
clear
C)
\[\alpha -\]particle
done
clear
D)
\[\beta -\]particle
done
clear
View Answer play_arrow
question_answer 52) In hydrogen atom, energy of first excited state is\[-3.4eV\]. Then \[KE\] of same orbit of hydrogen atom is
A)
\[+3.4eV\]
done
clear
B)
\[+6.8eV\]
done
clear
C)
\[-13.6eV\]
done
clear
D)
\[+13.6eV\]
done
clear
View Answer play_arrow
question_answer 53) Reaction\[Ba{{O}_{2}}(s)BaO(s)+{{O}_{2}}(g)\];\[\Delta H=+ve\]. In equilibrium condition, pressure of\[{{O}_{2}}\]depends on
A)
increased mass of \[Ba{{O}_{2}}\]
done
clear
B)
increased mass of\[BaO\]
done
clear
C)
increased temperature of equilibrium.
done
clear
D)
increased mass of\[Ba{{O}_{2}}\]and\[BaO\]both
done
clear
View Answer play_arrow
question_answer 54) Solubility of\[M{{X}_{2}}-\]type electrolytes is\[0.5\times {{10}^{-4}}mol/L\] Then find out \[{{K}_{sp}}\] of electrolytes
A)
\[5\times {{10}^{-12}}\]
done
clear
B)
\[25\times {{10}^{-10}}\]
done
clear
C)
\[1\times {{10}^{-13}}\]
done
clear
D)
\[5\times {{10}^{-13}}\]
done
clear
View Answer play_arrow
question_answer 55) \[1M\] and \[2.5\,\,L\,\,NaOH\] solution mixed with another \[0.5M\] and \[3\,\,L\,\,NaOH\] solution. Then find out molarity of resultant solution
A)
\[0.80M\]
done
clear
B)
\[1.0M\]
done
clear
C)
\[0.73M\]
done
clear
D)
\[0.50M\]
done
clear
View Answer play_arrow
question_answer 56) Which has highest pH?
A)
\[C{{H}_{3}}COOK\]
done
clear
B)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
C)
\[N{{H}_{4}}Cl\]
done
clear
D)
\[NaN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 57) Solution of \[0.1\,\,N\,\,N{{H}_{4}}OH\] and \[0.1\,\,N\,\,N{{H}_{4}}Cl\] has\[pH\,\,9.25\], then find out \[p{{K}_{b}}\]of\[N{{H}_{4}}OH\].
A)
\[9.25\]
done
clear
B)
\[4.75\]
done
clear
C)
\[3.75\]
done
clear
D)
\[8.25\]
done
clear
View Answer play_arrow
question_answer 58) van der Waals real gas, act as an ideal gas, at which condition?
A)
High temperature, low pressure
done
clear
B)
Low temperature, high pressure
done
clear
C)
High temperature, high pressure
done
clear
D)
Low temperature, low pressure
done
clear
View Answer play_arrow
question_answer 59) Unit of entropy is
A)
\[J{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
B)
\[J\,\,mo{{l}^{-1}}\]
done
clear
C)
\[{{J}^{-1}}{{K}^{-1}}\,\,mo{{l}^{-1}}\]
done
clear
D)
\[JK\,\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 60) In a closed insulated container a liquid is stirred with a paddle to increase the temperature, which of the following is true?
A)
\[\Delta E=\Delta W\ne 0,\,\,q=0\]
done
clear
B)
\[\Delta E=W=0,\,\,q\ne 0\]
done
clear
C)
\[\Delta E=0,\,\,W=q\ne 0\]
done
clear
D)
\[W=0,\,\,\Delta E=q\ne 0\]
done
clear
View Answer play_arrow
question_answer 61) 2 mole of ideal gas at \[{{27}^{o}}C\] temperature is expanded reversibly from \[2\,\,L\] to\[20\,\,L\]. Find entropy change\[(R=2cal/mol\,\,K)\]
A)
\[92.1\]
done
clear
B)
\[0\]
done
clear
C)
\[4\]
done
clear
D)
\[9.2\]
done
clear
View Answer play_arrow
question_answer 62) Heat of combustion \[\Delta H{}^\circ \] for\[C(s)\], \[{{H}_{2}}(g)\] and \[C{{H}_{4}}(g)\]are \[-94,\,\,-68\] and\[-213\,\,kcal/mol\]. Then\[\Delta H{}^\circ \]for\[C(s)+2{{H}_{2}}(g)\xrightarrow{{}}C{{H}_{4}}(g)\]is
A)
\[-17kcal\]
done
clear
B)
\[-111kcal\]
done
clear
C)
\[-170kcal\]
done
clear
D)
\[-85kcal\]
done
clear
View Answer play_arrow
question_answer 63) \[3A\xrightarrow{{}}2B\], rate of reactions\[+\frac{d[B]}{dt}\] is equal to
A)
\[-\frac{3}{2}\frac{d[A]}{dt}\]
done
clear
B)
\[-\frac{2}{3}\frac{d[A]}{dt}\]
done
clear
C)
\[-\frac{1}{3}\frac{d[A]}{dt}\]
done
clear
D)
\[+2\frac{d[A]}{dt}\]
done
clear
View Answer play_arrow
question_answer 64) \[3A\xrightarrow{{}}B+C\] It would be a zero order reaction when
A)
the rate of reaction is proportional to square of concentration of A
done
clear
B)
the rate of reaction remains same at any concentration of A
done
clear
C)
the rate remains unchanged at any concentration of B and C
done
clear
D)
the rate of reaction doubles if concentration of B is increased to double
done
clear
View Answer play_arrow
question_answer 65) Which has maximum molecules?
A)
\[7g{{N}_{2}}\]
done
clear
B)
\[2g{{H}_{2}}\]
done
clear
C)
\[16gN{{O}_{2}}\]
done
clear
D)
\[16g{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 66) A solution contains non-volatile solute of molecular mass\[{{M}_{2}}\]. Which of the following can be used to calculate the molecular mass of solute in terms of osmotic pressure?
A)
\[{{M}_{2}}=\left[ \frac{{{m}_{2}}}{\pi } \right]VRT\]
done
clear
B)
\[{{M}_{2}}=\left[ \frac{{{m}_{2}}}{V} \right]\frac{RT}{\pi }\]
done
clear
C)
\[{{M}_{2}}=\left[ \frac{{{m}_{2}}}{V} \right]\pi RT\]
done
clear
D)
\[{{M}_{2}}\left[ \frac{{{m}_{2}}}{V} \right]\frac{\pi }{RT}\]
done
clear
View Answer play_arrow
question_answer 67) A solution containing components A and B follows Raoults law
A)
\[A-B\] attraction force is greater than \[A-A\] and\[B-B\]
done
clear
B)
\[A-B\]attraction force is less than \[AA\] and\[B-B\]
done
clear
C)
\[A-B\]attraction force remains same as \[A-A\] and\[B-B\]
done
clear
D)
volume of solution is different from sum of volumes of solute and solvent
done
clear
View Answer play_arrow
question_answer 68) Which reaction is not feasible?
A)
\[2Kl+B{{r}_{2}}\to 3KBr+{{I}_{2}}\]
done
clear
B)
\[2KBr+{{I}_{2}}\to 2Kl+B{{r}_{2}}\]
done
clear
C)
\[2KBr+C{{l}_{2}}\to 2KCl+B{{r}_{2}}\]
done
clear
D)
\[2{{H}_{2}}O+2{{F}_{2}}\to 4HF+{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 69) In electrolysis of \[NaCI\] when \[Pt\] electrode is taken then \[{{H}_{2}}\] is liberated at. cathode while with \[Hg\] cathode it forms sodium amalgam
A)
Hg is more inert than\[Pt\]
done
clear
B)
more voltage is required to reduce \[{{H}^{+}}\] at \[Hg\] than at\[Pt\]
done
clear
C)
\[Na\] is dissolved in \[Hg\] while it does not dissolved in\[Pt\]
done
clear
D)
concentration of \[{{H}^{+}}\] ions is larger when \[Pt\] electrode is taken
done
clear
View Answer play_arrow
question_answer 70) Which of the following statement is true?
A)
Silicon exhibits 4 coordination number in its compounds
done
clear
B)
Bond energy of \[{{F}_{2}}\] is less than\[C{{l}_{2}}\]
done
clear
C)
Mn(III) oxidation state is more stable than Mn(II) in aqueous state
done
clear
D)
Elements of 15th group shows only+3 and +5 oxidation states
done
clear
View Answer play_arrow
question_answer 71) Which of the following order is wrong?
A)
\[N{{H}_{3}}<P{{H}_{3}}<As{{H}_{3}}-acidic\]
done
clear
B)
\[Li<Be<B<C-1st\,\,IP\]
done
clear
C)
\[A{{l}_{2}}{{O}_{3}}<MgO<N{{a}_{2}}O<{{K}_{2}}O-basic\]
done
clear
D)
\[L{{i}^{+}}<N{{a}^{+}}<{{K}^{+}}<C{{s}^{+}}-ionic\,\,radius\]
done
clear
View Answer play_arrow
question_answer 72) General electronic configuration of lanthanides are
A)
\[(n-2){{f}^{1-14}}(n-1){{s}^{2}}{{p}^{6}}{{d}^{0-1}}n{{s}^{2}}\]
done
clear
B)
\[(n-2){{f}^{10-14}}(n-1){{d}^{0-1}}n{{s}^{2}}\]
done
clear
C)
\[(n-2){{f}^{0-14}}(n-1){{d}^{10}}n{{s}^{2}}\]
done
clear
D)
\[(n-2){{d}^{0-1}}(n-1){{f}^{1-14}}n{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 73) An atom has electronic configuration\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{3}}4{{s}^{2}}\], you will place it in
A)
fifth group
done
clear
B)
fifteenth group
done
clear
C)
second group
done
clear
D)
third group
done
clear
View Answer play_arrow
question_answer 74) Which of the following is iso-electronic?
A)
\[C{{O}_{2}},\,\,N{{O}_{2}}\]
done
clear
B)
\[NO_{2}^{-},\,\,C{{O}_{2}}\]
done
clear
C)
\[C{{N}^{-}},\,\,CO\]
done
clear
D)
\[S{{O}_{2}},\,\,C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 75) Which of the following has\[{{p}_{\pi }}-{{d}_{\pi }}\], bonding?
A)
\[NO_{3}^{-}\]
done
clear
B)
\[SO_{3}^{2-}\]
done
clear
C)
\[BO_{3}^{3-}\]
done
clear
D)
\[CO_{3}^{2-}\]
done
clear
View Answer play_arrow
question_answer 76) In\[NO_{3}^{-}\]ion number of bond pair and lone pair of electron on nitrogen atom are
A)
2, 2
done
clear
B)
3, 1
done
clear
C)
1, 3
done
clear
D)
4, 0
done
clear
View Answer play_arrow
question_answer 77) Which of the following shows maximum number of oxidation states?
A)
\[Cr\]
done
clear
B)
\[Fe\]
done
clear
C)
\[Mn\]
done
clear
D)
\[V\]
done
clear
View Answer play_arrow
question_answer 78) Atomic number of \[Cr\] and \[Fe\] are respectively 24 and 26, which of the following is paramagnetic with the spin of electron?
A)
\[[Cr{{(CO)}_{6}}]\]
done
clear
B)
\[[Fe{{(CO)}_{5}}]\]
done
clear
C)
\[{{[Fe{{(CN)}_{6}}]}^{4-}}\]
done
clear
D)
\[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 79) The hypothetical complex chloro diaquatriammine cobalt (III) chloride can be represented as
A)
\[[CoCl{{(N{{H}_{3}})}_{3}}{{({{H}_{2}}O)}_{2}}]C{{l}_{2}}\]
done
clear
B)
\[[Co{{(N{{H}_{3}})}_{3}}({{H}_{2}}O)C{{l}_{3}}]\]
done
clear
C)
\[[Co{{(N{{H}_{2}})}_{3}}{{({{H}_{2}}O)}_{2}}Cl]\]
done
clear
D)
\[[Co{{(N{{H}_{3}})}_{3}}{{({{H}_{2}}O)}_{3}}]C{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 80) In the silver plating of copper, \[K[Ag{{(CN)}_{2}}]\] is used instead of\[AgN{{O}_{3}}\]. The reason is
A)
a thin layer of \[Ag\] is formed on \[Cu\]
done
clear
B)
more voltage is required
done
clear
C)
\[A{{g}^{+}}\] ions are completely removed from solution
done
clear
D)
less availability of \[A{{g}^{+}}\] ions, as \[Cu\] cannot displace \[Ag\] from\[{{[Ag{{(CN)}_{2}}]}^{-}}\]ion
done
clear
View Answer play_arrow
question_answer 81) \[CuS{{O}_{4}}\] when reacts with \[KCN\] forms \[CuCN\]. Which is insoluble in water. It is soluble in excess of \[KCN\], due to formation of the following complex
A)
\[{{K}_{2}}[Cu{{(CN)}_{4}}]\]
done
clear
B)
\[[{{K}_{2}}{{[CuCn)}_{4}}]\]
done
clear
C)
\[CuC{{N}_{2}}\]
done
clear
D)
\[Cu[K\,\,Cu{{(CN)}_{4}}]\]
done
clear
View Answer play_arrow
question_answer 82) Position of non-polar and polar parts in micelles
A)
polar at outer surface but non-polar at inner surface
done
clear
B)
polar at inner surface non-polar at outer surface
done
clear
C)
distributed over all the surface
done
clear
D)
are present in the surface only
done
clear
View Answer play_arrow
question_answer 83) In borax bead test, which compound is formed?
A)
ortho borate
done
clear
B)
meta borate
done
clear
C)
double oxide
done
clear
D)
tetra borate
done
clear
View Answer play_arrow
question_answer 84) \[Zn\]gives \[{{H}_{2}}\] gas with \[{{H}_{2}}S{{O}_{4}}\] and \[HCl\] but not with \[HN{{O}_{3}}\]
A)
\[Zn\]act as oxidising agent when react with\[HN{{O}_{3}}\]
done
clear
B)
\[HN{{O}_{3}}\]is weaker acid than \[{{H}_{2}}S{{O}_{4}}\] and \[HCl\]
done
clear
C)
In electrochemical series \[Zn\] is above hydrogen
done
clear
D)
\[NO_{3}^{-}\] is reduced in preference to hydronium ion
done
clear
View Answer play_arrow
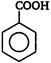
question_answer 85) \[IUPAC\]name of the following is\[C{{H}_{2}}=CH-C{{H}_{2}}-C{{H}_{2}}-C\equiv CH\]
A)
1, 5-hexenyne
done
clear
B)
1-hexene-5-yne
done
clear
C)
1-hexyne-5-ene
done
clear
D)
1, 5-hexynene
done
clear
View Answer play_arrow
question_answer 86)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 87) \[n-\]propyl alcohol and isopropyl alcohol can be chemically distinguished by which reagent
A)
PCIs
done
clear
B)
reduction
done
clear
C)
oxidation with potassium dichromate
done
clear
D)
ozonolysis
done
clear
View Answer play_arrow
question_answer 88) In the following reaction product\[P\]is\[R-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-Cl\xrightarrow[Pd-BaS{{O}_{4}}]{{{H}_{2}}}p\]
A)
\[RC{{H}_{2}}OH\]
done
clear
B)
\[RCOOH\]
done
clear
C)
\[RCHO\]
done
clear
D)
\[RC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 89) \[\overset{\centerdot \,\,\,\centerdot }{\mathop{C{{H}_{2}}}}\,-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\]and\[C{{H}_{2}}=\underset{\begin{smallmatrix} || \\ :O: \\ \,\centerdot \,\,\centerdot \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\]
A)
resonating structures
done
clear
B)
tautomers
done
clear
C)
geometrical isomers
done
clear
D)
optical isomers
done
clear
View Answer play_arrow
question_answer 90) Reactivity order of halides for dehydrohalogenation is
A)
\[R-F>R-Cl>R-Br>R-I\]
done
clear
B)
\[R-I>R-Br>R-Cl>R-F\]
done
clear
C)
\[R-I>R-Cl>R-Br>R-F\]
done
clear
D)
\[R-F>R-I>R-Br>R-Cl\]
done
clear
View Answer play_arrow
question_answer 91) Monomer of\[{{\left[ -\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{2}}- \right]}_{n}}\]is
A)
2-methyl propene
done
clear
B)
styrene
done
clear
C)
propylene
done
clear
D)
ethane
done
clear
View Answer play_arrow
question_answer 92)
A)
done
clear
B)
done
clear
C)
done
clear
D)
\[{{C}_{6}}{{H}_{5}}-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-{{C}_{6}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 93) Cellulose is polymer of
A)
glucose
done
clear
B)
fructose
done
clear
C)
ribose
done
clear
D)
sucrose
done
clear
View Answer play_arrow
question_answer 94) \[C{{H}_{3}}C{{H}_{2}}Cl\xrightarrow{NaCN}X\xrightarrow{Ni/{{H}_{2}}}Y\]\[Z\xleftarrow{Aceticanhydride}\]in above reaction sequence, \[Z\]is
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}NHCOC{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CONHC{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CONHCOC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 95) When phenol is treated with \[CHC{{l}_{3}}\] and \[NaOH\] the product formed is
A)
benzaldehyde
done
clear
B)
salicylaldehyde
done
clear
C)
salicylic acid
done
clear
D)
benzoicacid
done
clear
View Answer play_arrow
question_answer 96) The percentage of \[C,\,\,H\] and \[N\] in an organic compound are \[40%,\,\,13.3%\] and \[46.7%\] respectively then empirical formula is
A)
\[{{C}_{3}}{{H}_{13}}{{N}_{3}}\]
done
clear
B)
\[C{{H}_{2}}N\]
done
clear
C)
\[C{{H}_{4}}N\]
done
clear
D)
\[C{{H}_{6}}N\]
done
clear
View Answer play_arrow
question_answer 97) Enzymes are made up of
A)
edible proteins
done
clear
B)
proteins with specific structure
done
clear
C)
nitrogen containing carbohydrates
done
clear
D)
carbohydrates
done
clear
View Answer play_arrow
question_answer 98) Geometrical isomers differ in
A)
position of functional group
done
clear
B)
position of atoms
done
clear
C)
spatial arrangement of atoms
done
clear
D)
length of carbon chain
done
clear
View Answer play_arrow
question_answer 99) When\[C{{H}_{3}}C{{H}_{2}}CHC{{l}_{2}}\]is treated with\[NaN{{H}_{2}}\], the product formed is
A)
\[C{{H}_{3}}-CH=C{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}-CH\equiv CH\]
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 100) Which is not true statement?
A)
\[\alpha -\]carbon of a-amino acid is asymmetric
done
clear
B)
All proteins are found in \[L-\]form
done
clear
C)
Human body can synthesise all proteins they need
done
clear
D)
At \[pH=7\] both amino and carboxylic groups exist in ionised form
done
clear
View Answer play_arrow
question_answer 101) Mullets fibres occur in which part?
A)
Heart
done
clear
B)
Kidney
done
clear
C)
Pancreas
done
clear
D)
Retina
done
clear
View Answer play_arrow
question_answer 102) A commonly used mastigator called supari is obtained from the plant
A)
Acacia catechu
done
clear
B)
Areca catechu
done
clear
C)
Pipe betel
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 103) The aril of litchi which is edible part is made up of:
A)
I integument
done
clear
B)
II integument
done
clear
C)
III integument
done
clear
D)
IV integument
done
clear
View Answer play_arrow
question_answer 104) A drug called morphine is obtained from
A)
Rauwolfia serpentine
done
clear
B)
Cannabis sativa
done
clear
C)
Cajanus cajan
done
clear
D)
Papaver somniferum
done
clear
View Answer play_arrow
question_answer 105) Epigynous flowers with numerous stamens are found in
A)
Ranunculus muricatus
done
clear
B)
Fragaria indica
done
clear
C)
Croton roxburghii
done
clear
D)
Syzygium cuminis
done
clear
View Answer play_arrow
question_answer 106) Interphase nucleus is enclosed by
A)
non-porous nuclear membrane
done
clear
B)
porous double nuclear membrane
done
clear
C)
non-porous double discontinuous nuclear membrane
done
clear
D)
a single porous unit membrane
done
clear
View Answer play_arrow
question_answer 107) Golgi apparatus: Transports and modifies material Secrete mucin in respiratory tract Secretes slime in insectivorous plants. What is correct?
A)
Wrong A, correct B and C
done
clear
B)
Wrong B, correct A and C
done
clear
C)
Wrong B and C, correct A
done
clear
D)
Wrong none, correct all
done
clear
View Answer play_arrow
question_answer 108) The lowest number of chromosomes is found in which of the following?
A)
Haplopappus gracilis
done
clear
B)
Poa litorosa
done
clear
C)
Salix tetrasperma
done
clear
D)
Ageratum coigzoides
done
clear
View Answer play_arrow
question_answer 109) The nucleotide sequence of an anticodon is complementary to nucleotide sequence in
A)
tRNA
done
clear
B)
mRNA
done
clear
C)
rRNA
done
clear
D)
DNA
done
clear
View Answer play_arrow
question_answer 110) Which one of the following amino acids can stabilize protein structure by forming disulphide bonds?
A)
Arginine
done
clear
B)
Lysine
done
clear
C)
Cysteine
done
clear
D)
Alanine
done
clear
View Answer play_arrow
question_answer 111) Chromosome number can be doubled by using which of the following?
A)
Indole acetic acid
done
clear
B)
GA
done
clear
C)
Zeatin
done
clear
D)
Colchicine
done
clear
View Answer play_arrow
question_answer 112) Dr. Karry B Mullis was awarded Nobel Prize in chemistry in 1993 for his work on
A)
site directed mutagenesis
done
clear
B)
polymerase chain reaction
done
clear
C)
mobile genetic elements
done
clear
D)
antibody diversity
done
clear
View Answer play_arrow
question_answer 113) Which of the following cell organelles is rich in catabolic enzymes?
A)
Chloroplast
done
clear
B)
Mitochondria
done
clear
C)
Golgi complex
done
clear
D)
Ribosomes
done
clear
View Answer play_arrow
question_answer 114) A conspicuous rounded body present in nucleoplasm and attached to a particular chromosome at a definite place is
A)
plasmid
done
clear
B)
karyolymph
done
clear
C)
nucleolus
done
clear
D)
nuclear reticulum
done
clear
View Answer play_arrow
question_answer 115) Nucleolus is
A)
rounded structure found in cytoplasm near nucleus
done
clear
B)
rounded structure inside nucleus and having rRNA
done
clear
C)
rod shaped structure in cytoplasm near the nucleus
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 116) Bt cotton contains genes from
A)
Bacillus thuringiensis
done
clear
B)
Bacillus thermos
done
clear
C)
Bacteroides thuringiensis
done
clear
D)
Boreuia tunicate
done
clear
View Answer play_arrow
question_answer 117) A drug obtained through genetic engineering and useful for treating infertility is
A)
calcitonin
done
clear
B)
chorionic gonadotropin
done
clear
C)
interleukin
done
clear
D)
tissue plasminogen activator
done
clear
View Answer play_arrow
question_answer 118) The biological concept of species was formulated by
A)
Mayr
done
clear
B)
Stebbins
done
clear
C)
Heywood
done
clear
D)
Love
done
clear
View Answer play_arrow
question_answer 119) The total number of angiospermous families in Bentham and Hookers system of classification is:
A)
205
done
clear
B)
203
done
clear
C)
201
done
clear
D)
200
done
clear
View Answer play_arrow
question_answer 120) Which of the following pairs of families possess pollinia?
A)
Orchidaceae and Apocynaceae
done
clear
B)
Orchidaceae and Asclepiadaceae
done
clear
C)
Asclepiadaceae and Mimosaceae
done
clear
D)
Asclepiadaceae and Apocynaceae
done
clear
View Answer play_arrow
question_answer 121) When the specific epithet exactly repeats, generic name. It is called as
A)
tautonym
done
clear
B)
synonym
done
clear
C)
basionym
done
clear
D)
homonym
done
clear
View Answer play_arrow
question_answer 122) Solution of polyethylene glycol (PEG) or a very brief high voltage electric current is used in fusion of
A)
protoplasms
done
clear
B)
protoplasts
done
clear
C)
somatic cells
done
clear
D)
germinal cells
done
clear
View Answer play_arrow
question_answer 123) The enzyme which converts corn starch into fructose rich corn syrup is
A)
amylases
done
clear
B)
glucoamylases
done
clear
C)
glucoisomerases
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 124) Wine and beer are produced directly by fermentation. Brandy and whisky require both fermentation and distillation because
A)
fermentation is inhibited at an alcohol level of 10-18%.
done
clear
B)
distillation prolongs storage
done
clear
C)
distillation improves quality
done
clear
D)
distillation purifies the beverage
done
clear
View Answer play_arrow
question_answer 125) The phenomenon of antibiosis (ie, secretion of one microbe inhibit the growth of other microbes) was discovered by
A)
Pasteur
done
clear
B)
Babes
done
clear
C)
Flemming
done
clear
D)
Waksman
done
clear
View Answer play_arrow
question_answer 126) A ring of multicilliate zoogonidium is found in
A)
Ulothrix
done
clear
B)
Zygnema
done
clear
C)
Oedogonium
done
clear
D)
Chara
done
clear
View Answer play_arrow
question_answer 127) The only living fossil which is known by the name of maiden hair tree
A)
Thuja
done
clear
B)
Pinus
done
clear
C)
Ginkgo
done
clear
D)
Araucaria
done
clear
View Answer play_arrow
question_answer 128) Which green algae shows hetero trichous habit and may have given rise to terrestrial (land) habit?
A)
Chlamydomonas
done
clear
B)
Fritschiella
done
clear
C)
Vaucheria
done
clear
D)
Ulothrix
done
clear
View Answer play_arrow
question_answer 129) A group of plants which are autotrophs, their sex organs are non-jacketed and whose zygotes secrete thick wall are called
A)
phycophytes
done
clear
B)
lichens
done
clear
C)
bryophytes
done
clear
D)
thallophytes
done
clear
View Answer play_arrow
question_answer 130)
Match the following ovular structure with post fertilization structure and select the correct alternative A. Ovule 1. Endosperm B. Funiculus 2. Aril C. Nucellus 3. Seed D. Polar nuclei 4. Perisperm
A)
A-2 B-3 C-4 D-1
done
clear
B)
A-2 B-3 C-1 D-4
done
clear
C)
A-3 B-2 C-4 D-1
done
clear
D)
A-3 B-2 C-1 D-4
done
clear
View Answer play_arrow
question_answer 131) The beri-beri is a paralytic disease caused by the deficiency of vitamin-Bi (thiamine). It was discovered by
A)
Funk
done
clear
B)
GE Foxon
done
clear
C)
Eijkman
done
clear
D)
Stanley
done
clear
View Answer play_arrow
question_answer 132) Which of the following pair is characterised by swollen lip, thick, pigmented skin of hands and legs are irritability?
A)
Thiamine - Beri-beri
done
clear
B)
Protein - Kwashiorkor
done
clear
C)
Nicotinamide - Pellagra
done
clear
D)
Iodine - Goitre
done
clear
View Answer play_arrow
question_answer 133) During prolonged fasting
A)
first fats are used by, followed by carbohydrates from liver and muscles and proteins in the end
done
clear
B)
first carbohydrates are used up, followed by fat and proteins towards ends
done
clear
C)
first lipid, followed by protein and carbohydrates towards ends
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 134) Lathyrism which results due to consumption of khebaridal is characterised by
A)
skeletal deformation and thinning of collagen fibre
done
clear
B)
skeletal abnormality, diabetes mellitus and reproductive failure
done
clear
C)
retailed growth precocious puberty and renal dysfunction
done
clear
D)
cardio vascular abnormalities, mental retardation and delayed puberty
done
clear
View Answer play_arrow
question_answer 135) The enzyme which converts maltose into glucose is
A)
diastase
done
clear
B)
hydrogenase
done
clear
C)
invertase
done
clear
D)
maltase
done
clear
View Answer play_arrow
question_answer 136)
Arrange the following in the order of increasing volume (1) Tidal volume (2) Residual volume (3) Expiratory reserve volume (4) Vital capacity
Codes:
A)
1 < 2 < 3 < 4
done
clear
B)
1 < 3 < 2 < 4
done
clear
C)
1 < 4 < 3 < 2
done
clear
D)
1 < 4 < 2 < 3
done
clear
View Answer play_arrow
question_answer 137) In a copulating pair of earthworm, which two process takes place?
A)
External fertilization and cross fertilization
done
clear
B)
Cross fertilization and reciprocal fertilization
done
clear
C)
Internal fertilization and cross fertilization
done
clear
D)
Reciprocal fertilization and internal fertilization
done
clear
View Answer play_arrow
question_answer 138) The part of spermatheca of earthworm that acts as store house of spermatozoa is
A)
ampulla
done
clear
B)
diverticulum
done
clear
C)
both [a] and [b]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 139) The correct arrangement of leg parts of cockroach is
A)
coxa, femur, trochanter, tibia and claws
done
clear
B)
coxa, trochanter, femur, tibia, tarsus and claws
done
clear
C)
coxa, tibia, femur, plantulae and claws
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 140) During respiration in frog the hyoid and floor of the buccal cavity are raised with the help of
A)
stemohyal muscles
done
clear
B)
petrohyal muscles
done
clear
C)
ligaments
done
clear
D)
inter coastal muscles
done
clear
View Answer play_arrow
question_answer 141) Jacobsons organs which are additional olfactory organ are present in
A)
rat
done
clear
B)
snakes
done
clear
C)
man
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 142) Right lung of rat has 4 lobes. The left lung has how many lobe/lobes?
A)
One
done
clear
B)
Two
done
clear
C)
Three
done
clear
D)
Four
done
clear
View Answer play_arrow
question_answer 143) In frogs heart, which of the following is considered as pacemaker?
A)
Pyrangium
done
clear
B)
Synangium
done
clear
C)
Sinus venosus
done
clear
D)
Truncus arteriosus
done
clear
View Answer play_arrow
question_answer 144) Eggs which have yolk in the centre surrounded by cytoplasm are called
A)
centrolecithal
done
clear
B)
homolecithal
done
clear
C)
microlecithal
done
clear
D)
alecithal
done
clear
View Answer play_arrow
question_answer 145) If an earthworm is left in \[40%\text{ }KOH\] solution for a long time, which part would be left undissolved?
A)
Setae
done
clear
B)
Sperma thecae
done
clear
C)
Sand particles
done
clear
D)
Circular muscles
done
clear
View Answer play_arrow
question_answer 146) Which of the following is the character of dorsal blood vessel of the earthworm?
A)
Collecting in the whole body
done
clear
B)
Collecting in first 13 segments
done
clear
C)
Distributing in the whole body
done
clear
D)
Distributing in the first 13 segments
done
clear
View Answer play_arrow
question_answer 147) The dorsal plate of skeleton found on the abdomen of cockroach is called
A)
pleuron
done
clear
B)
sternum
done
clear
C)
tergum
done
clear
D)
vertex
done
clear
View Answer play_arrow
question_answer 148) A normal plant suddenly started reproducing partheno genetically. The miffiber of chromosomes of the second generation as compared to the parent will be
A)
one half
done
clear
B)
one fourth
done
clear
C)
same
done
clear
D)
double
done
clear
View Answer play_arrow
question_answer 149) The number of punctuation codons in a genetic code are
A)
\[2+3=5\]
done
clear
B)
\[1+3=4\]
done
clear
C)
\[1+1=2\]
done
clear
D)
\[3\]
done
clear
View Answer play_arrow
question_answer 150) Out of the following which is a genetically engineered antiviral protein?
A)
Humulin
done
clear
B)
Interferon
done
clear
C)
Fumagillin
done
clear
D)
Griseofulvin
done
clear
View Answer play_arrow
question_answer 151) The number of chromosomes in endospermic cell is 36. What will be the number of chromosome in root tip cells?
A)
9
done
clear
B)
18
done
clear
C)
12
done
clear
D)
24
done
clear
View Answer play_arrow
question_answer 152) Phragmoplast is
A)
proplasted in cytoplasm of dividing cells
done
clear
B)
cell plate formed by vesicles of ER and dictyosomes during cytokinesis
done
clear
C)
cell plate formed by ER, dictyosomes secretory vesicles and spindle fibre
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 153) Mitosis is a process by which eukaryotic cells
A)
grow
done
clear
B)
get specialised in structure
done
clear
C)
multiply
done
clear
D)
expose the genes
done
clear
View Answer play_arrow
question_answer 154) The absorption spectrum of chlorophyll
A)
shows that some colours of light are absorbed more than the others
done
clear
B)
approximates the action spectrum of photosynthesis
done
clear
C)
explains why chlorophyll is a green pigment
done
clear
D)
has all the above properties
done
clear
View Answer play_arrow
question_answer 155) Which of the following pairs of chromosomal mutation are most likely to occur when homologous chromosomes are undergoing synapsis?
A)
Deletion and inversion
done
clear
B)
Duplication and translocation
done
clear
C)
Deletion and duplication
done
clear
D)
Inversion and translocation
done
clear
View Answer play_arrow
question_answer 156) Interferon is a type of protein which is used to cure
A)
homeostatic disorder
done
clear
B)
hepatitis caused by virus
done
clear
C)
common cold caused by virus
done
clear
D)
both b and c
done
clear
View Answer play_arrow
question_answer 157) Bacteria bearing tufts of flagella on both the poles are known as
A)
peritrichous
done
clear
B)
cephalotrichous
done
clear
C)
lophotrichous
done
clear
D)
amphitrichous
done
clear
View Answer play_arrow
question_answer 158) Antigen binds to antibody. This binding is a result of
A)
electrostatic interactions
done
clear
B)
covalent bonds
done
clear
C)
disulphide bridges
done
clear
D)
amide formation
done
clear
View Answer play_arrow
question_answer 159) An \[R{{h}^{-}}\] individual receives \[R{{h}^{+}}\] blood. The recipient becomes
A)
sterile
done
clear
B)
dead
done
clear
C)
no reaction
done
clear
D)
isoimmunised
done
clear
View Answer play_arrow
question_answer 160) Who isolated rennet a cheese producting enzyme from calfs stomach?
A)
Flemming
done
clear
B)
Hensen
done
clear
C)
Waksman
done
clear
D)
Smith and Norths
done
clear
View Answer play_arrow
question_answer 161) The missing link between amphibians and reptiles is
A)
Archaeopteryx
done
clear
B)
Ichthyostega
done
clear
C)
Seymouria
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 162) In a conditions when potential competitors accidently come together to share the same living place, extreme types from each population, those who might complete least are favoured, is a phenomenon called
A)
competitive exclusion
done
clear
B)
character displacement
done
clear
C)
commensalism
done
clear
D)
mimicry
done
clear
View Answer play_arrow
question_answer 163) Evolutionary change does not comes about at the level of individual but at the level of
A)
two persons
done
clear
B)
10 persons
done
clear
C)
population
done
clear
D)
small group
done
clear
View Answer play_arrow
question_answer 164) Population-I and II are growing in the same habitat and are morphologically similar but these are intersterile. In terms of biological concept of species they may be considered as
A)
one species
done
clear
B)
two distinct species
done
clear
C)
two siblings species
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 165) The biological definition of a species depends on
A)
the geographical distribution of two groups of organisms
done
clear
B)
reproductive isolation of two groups of organisms
done
clear
C)
anatomical and developmental differences between the two groups of organisms
done
clear
D)
difference in the adaptation of two groups of organisms
done
clear
View Answer play_arrow
question_answer 166) Which of the following is controlled by multiple alleles?
A)
Sickle cell anaemia
done
clear
B)
Colour blindness
done
clear
C)
Blood groups
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 167) Descent with modification is the main theme of
A)
genetics and interpretation
done
clear
B)
biogenesis
done
clear
C)
recapitulation
done
clear
D)
evolution
done
clear
View Answer play_arrow
question_answer 168) What percentage of solar radiation that hits the earths atmosphere ever reaches the surface?
A)
92%
done
clear
B)
2%
done
clear
C)
42%
done
clear
D)
22%
done
clear
View Answer play_arrow
question_answer 169) The niche of a population is defined as
A)
set of condition that interacts
done
clear
B)
place where it lives
done
clear
C)
set of conditions and resources it uses
done
clear
D)
geographical area that it coveres
done
clear
View Answer play_arrow
question_answer 170) The reflectivity percentage of incident light on earth is meterologi cally called as
A)
tornado
done
clear
B)
albedo
done
clear
C)
refraction
done
clear
D)
reradiation
done
clear
View Answer play_arrow
question_answer 171) A high density of tiger population in an area can result in
A)
predation
done
clear
B)
interspecific (internecine) competition
done
clear
C)
intraspecific (intranecine) competition
done
clear
D)
proto co-operation
done
clear
View Answer play_arrow
question_answer 172) Pond is defined as a
A)
biome
done
clear
B)
agroecosystems
done
clear
C)
natural ecosystem
done
clear
D)
community
done
clear
View Answer play_arrow
question_answer 173) Maximum amount of oxygen is obtained from
A)
phytoplankton
done
clear
B)
grasslands
done
clear
C)
forests
done
clear
D)
herbs and shrubs
done
clear
View Answer play_arrow
question_answer 174) In many countries spread of Opuntia was controlled by
A)
DDT
done
clear
B)
growing trees
done
clear
C)
introducing insects
done
clear
D)
letting lose catties
done
clear
View Answer play_arrow
question_answer 175) Of the following four metropolitan Indian cities, where polluted air hangs above like a cloud
A)
Mumbai
done
clear
B)
Delhi
done
clear
C)
Kolkata
done
clear
D)
Chennai
done
clear
View Answer play_arrow
question_answer 176) Number of endangered species of angiosperms in India is
A)
487
done
clear
B)
15,000
done
clear
C)
5,000
done
clear
D)
3,000
done
clear
View Answer play_arrow
question_answer 177) The vascular bundle, where the phloem is surrounded by xylem is known as
A)
amphivasal
done
clear
B)
bicollateral
done
clear
C)
amphicribal
done
clear
D)
radial
done
clear
View Answer play_arrow
question_answer 178) Interxylary as well as intraxylary phloem is present in
A)
Bignonia
done
clear
B)
Mirdbilis
done
clear
C)
Strychnos
done
clear
D)
Achyranthes
done
clear
View Answer play_arrow
question_answer 179) In grana, of chloroplast, the reaction ADP + Pi = ATP during day shows
A)
oxidative phosphorylation
done
clear
B)
photophosphorylation
done
clear
C)
substrate level phosphorylation
done
clear
D)
dephosphorylation
done
clear
View Answer play_arrow
question_answer 180) Out of the given cell organelle which does not possess DNA?
A)
Peroxisome
done
clear
B)
Chloroplast
done
clear
C)
Mitochondria
done
clear
D)
Nucleus
done
clear
View Answer play_arrow
question_answer 181) Imbibition involves
A)
diffusion of water
done
clear
B)
movement of water into imbibant through capillary
done
clear
C)
movement of water into imbibant through diffusion as well as capillary action
done
clear
D)
absorption of water
done
clear
View Answer play_arrow
question_answer 182) On the basis of symptoms of chlorosis in leaves a student inferred that this was due to deficiency of nitrogen. This inference could be correct only if we assume that yellowing of leaves appeared first in
A)
old leaves
done
clear
B)
young leaves
done
clear
C)
young leaves followed by mature leaves
done
clear
D)
mature leaves followed by young leaves
done
clear
View Answer play_arrow
question_answer 183) Basic features of Kranz anatomy of \[{{C}_{4}}\] plant is
A)
presence of chloroplast in bundle sheath cells
done
clear
B)
presence of chloroplast in mesophyll and epidermal cells
done
clear
C)
presence of typical granal chloroplasts in bundle sheath cells and rudimentary chloroplast in mesophyll cells
done
clear
D)
presence of rudimentary chloroplasts in bundle sheath cells and typical granal chloroplasts in mesophyll cells
done
clear
View Answer play_arrow
question_answer 184) \[2NADH({{H}^{+}})\]produced during anaerobic glycolysis yield
A)
6 ATP molecules
done
clear
B)
4 ATP molecules
done
clear
C)
8 ATP molecules
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 185) In photo respiration which is light induced cyclic oxidation of photosynthetic intermediates with the help of oxygen, the substrate is
A)
glycolate
done
clear
B)
glucose
done
clear
C)
pyruvicacid
done
clear
D)
acetyl Co-A
done
clear
View Answer play_arrow
question_answer 186) RQ value of 4 may be expected for the complete oxidation of which one of the following?
A)
Glucose
done
clear
B)
Malic acid
done
clear
C)
Oxalic acid
done
clear
D)
Tartaric acid
done
clear
View Answer play_arrow
question_answer 187) When pea seeds and wheat seeds are put in water, which of the two will imbibe more water?
A)
Wheat seeds
done
clear
B)
Pea seeds
done
clear
C)
Both will imbibe equal amount of water
done
clear
D)
Pea seeds imbibe water only at alkaline pH
done
clear
View Answer play_arrow
question_answer 188) Who proved for the first time that the plants contain a large number of minerals and micro elements?
A)
De Saussure (1804)
done
clear
B)
Leibeg (1840)
done
clear
C)
Glauber and Mayhon (1650)
done
clear
D)
Amon and Stout (1939)
done
clear
View Answer play_arrow
question_answer 189) The rate of diffusion is dependent upon the permeability of the medium, it however
A)
influences the final equilibrium of diffusion as it is never reached if the medium is dense
done
clear
B)
does influences the final equilibrium of diffusion
done
clear
C)
does not influences the final equilibrium of diffusion
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 190) Hydroponics is a system of growing plants in
A)
soil less cultures or solution culture
done
clear
B)
acidic soils
done
clear
C)
soil less culture with alkaline pH
done
clear
D)
soil less culture with acidic pH
done
clear
View Answer play_arrow
question_answer 191) Sometimes there are more than two alleles for a given chromosome locus in this case a trait is controlled by
A)
co dominance
done
clear
B)
pseudo dominance
done
clear
C)
incomplete dominance
done
clear
D)
multiple alleles
done
clear
View Answer play_arrow
question_answer 192) Klinefeltefs syndrome is describe as a condition in human individual when sex chromosome constitution is
A)
XYO
done
clear
B)
XXX
done
clear
C)
XXO
done
clear
D)
XXY
done
clear
View Answer play_arrow
question_answer 193) Person suffering from disease phenylketonuria which is autosomal recessive disease lack which of these?
A)
Homogentisic acid
done
clear
B)
Phenyl alanine hydroxylase
done
clear
C)
Caeruloplasmin
done
clear
D)
Cystine
done
clear
View Answer play_arrow
question_answer 194) If the ratio between X-chromosomes and complete set of autosome is 0.5. Then the individual will be
A)
female
done
clear
B)
super female
done
clear
C)
male
done
clear
D)
super male
done
clear
View Answer play_arrow
question_answer 195) According to genie balance theory which was given by CB Bridges the sex of Drosophila is determined by
A)
ratio between the number of Y-chromosomes and complete set of autosomes
done
clear
B)
ratio between the number of X-chromosome and complete set of autosomes
done
clear
C)
both are correct
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 196) Genie balance theory of sex determination stated by CB Bridges is related to
A)
Drosophila melanogaster
done
clear
B)
Rumex
done
clear
C)
Snapdragon
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 197) Genes when present in homozygous condition results in non-viable progeny the factor responsible for such conditions are
A)
polygenes
done
clear
B)
linked genes
done
clear
C)
lethal genes
done
clear
D)
epistatic genes
done
clear
View Answer play_arrow
question_answer 198) Haemophilia, a X-linked recessive disease is caused due to deficiency of
A)
blood plasma and vitamin-K
done
clear
B)
blood platelets and haemoglobin
done
clear
C)
lack of clotting material and vitamin-K
done
clear
D)
allot the above
done
clear
View Answer play_arrow
question_answer 199) Wild type of cells which are unable to grow on minimal media and nutritional mutants are respectively known as
A)
prototrophs, auxophytes
done
clear
B)
auxotrophs, prototrophs
done
clear
C)
prototypes and auxotrophs
done
clear
D)
prototrophs and auxotrophs
done
clear
View Answer play_arrow
question_answer 200) The plant which is used for studying hybrid vigour or heterosis is
A)
maize
done
clear
B)
pea
done
clear
C)
datura
done
clear
D)
none of the above
done
clear
View Answer play_arrow
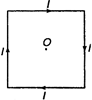
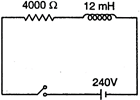
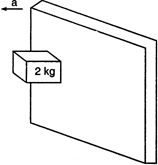
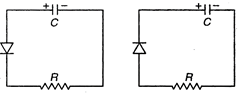
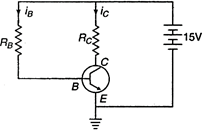
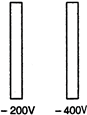
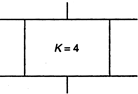
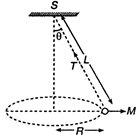
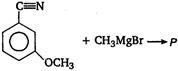 Product \[P\] in the above reaction is
Product \[P\] in the above reaction is
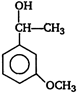
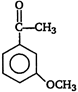
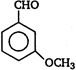
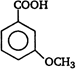
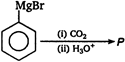 In the above reaction product P is
In the above reaction product P is