question_answer 1) The dimension \[[M{{L}^{-1}}{{T}^{-2}}]\] represents
A)
Youngs modulus
done
clear
B)
stress
done
clear
C)
pressure
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 2) A cylinder of mass \[10\,\,kg\] is rolling on a plane with an initial velocity of\[10\,\,m/s\]. If coefficient of friction between surface and cylinder is\[0.5\], then before stopping, it will describe
A)
\[12.5\,\,m\]
done
clear
B)
\[5\,\,m\]
done
clear
C)
\[7.5\,\,m\]
done
clear
D)
\[10\,\,m\]
done
clear
View Answer play_arrow
question_answer 3) A bomber plane moves horizontally with a speed of \[500\,\,m/s\] and bomb released from it strikes the ground in\[10\sec \]. Angle at which it strikes the ground will be\[(g=10m/{{s}^{2}})\]
A)
\[{{\tan }^{-1}}\frac{1}{5}\]
done
clear
B)
\[{{\tan }^{+1}}\frac{1}{5}\]
done
clear
C)
\[{{\tan }^{-1}}1\]
done
clear
D)
\[{{\tan }^{-1}}5\]
done
clear
View Answer play_arrow
question_answer 4) A body of mass \[40\,\,kg\] having velocity \[4\,\,m/s\] colloids with another body of mass 60kg having velocity\[2\,\,m/s\]. If the collision is inelastic, then loss in kinetic energy will be
A)
\[440\,\,\text{J}\]
done
clear
B)
\[392\,\,\text{J}\]
done
clear
C)
\[48\,\,\text{J}\]
done
clear
D)
\[144\,\,\text{J}\]
done
clear
View Answer play_arrow
question_answer 5) The apparent weight of the body, when it is travelling downwards with an acceleration of \[2\,\,m/s\] and mass is\[10\,\,kg\], will be
A)
\[198\,\,N\]
done
clear
B)
\[164\,\,N\]
done
clear
C)
\[140\,\,N\]
done
clear
D)
\[118\,\,N\]
done
clear
View Answer play_arrow
question_answer 6) A cyclist moves in a circular track or radius\[100\,\,m\]. If the coefficient of friction\[0.2\]. Then the maximum speed with which the cyclist can take a turn without leaving inwards, is
A)
\[14.0\,\,m/s\]
done
clear
B)
\[140\,\,m/s\]
done
clear
C)
\[1.4\,\,m/s\]
done
clear
D)
\[9.8\,\,m/s\]
done
clear
View Answer play_arrow
question_answer 7) The horizontal range of a projectile is \[4\sqrt{3}\] times its maximum height, then the angle of projection is
A)
\[{{30}^{o}}\]
done
clear
B)
\[{{45}^{o}}\]
done
clear
C)
\[{{60}^{o}}\]
done
clear
D)
\[{{90}^{o}}\]
done
clear
View Answer play_arrow
question_answer 8) A block slides down an inclined plane making an angle \[\theta ={{30}^{o}}\] with the horizontal with an acceleration\[\frac{g}{4}\], then coefficient of frictions will be
A)
\[\frac{1}{4}\left( \frac{\sqrt{3}}{2} \right)\]
done
clear
B)
\[\frac{1}{4}-\frac{\sqrt{3}}{2}\]
done
clear
C)
\[\frac{1}{2\sqrt{3}}\]
done
clear
D)
\[2\sqrt{3}\]
done
clear
View Answer play_arrow
question_answer 9) A wheel of mass \[10\,\,kg\] has a moment of inertia of \[160\,\,kg\text{-}{{m}^{2}}\] about its own axis, the radius of gyration will be
A)
\[10\,\,m\]
done
clear
B)
\[8\,\,m\]
done
clear
C)
\[6\,\,m\]
done
clear
D)
\[4\,\,m\]
done
clear
View Answer play_arrow
question_answer 10) A running man has half the kinetic energy of that of a boy of half of his mass. The man speeds up by \[1\,\,m/s\] so as to have same K.E. as that of the boy. The original speed of the man will be
A)
\[\sqrt{2}\,\,m/s\]
done
clear
B)
\[(\sqrt{2}-1)\,\,m/s\]
done
clear
C)
\[\frac{1}{(\sqrt{2}-1)}\,\,m/s\]
done
clear
D)
\[\frac{1}{\sqrt{2}}\,\,m/s\]
done
clear
View Answer play_arrow
question_answer 11) If a cycle wheel of radius \[4\,\,m\] completes one revolution in two seconds. Then acceleration of the cycle will be
A)
\[{{\pi }^{2}}m/{{s}^{2}}\]
done
clear
B)
\[2{{\pi }^{2}}m/{{s}^{2}}\]
done
clear
C)
\[4{{\pi }^{2}}m/{{s}^{2}}\]
done
clear
D)
\[8\pi \,\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 12) Assuming earth to be a sphere of a uniform density, the value of g in a mine 100 km below the earth surface will be
A)
\[3.66\,\,m/{{s}^{2}}\]
done
clear
B)
\[5.66\,\,m/{{s}^{2}}\]
done
clear
C)
\[7.64\,\,m/{{s}^{2}}\]
done
clear
D)
\[9.66\,\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 13) The angular speed of earth, so that the object on equator may appear weight less, is \[(g=10)\,\,m/{{s}^{2}}\], radius of earth\[6400\,\,km\]
A)
\[1.25\times {{10}^{-3}}rad/s\]
done
clear
B)
\[1.56\times {{10}^{-3}}rad/\sec \]
done
clear
C)
\[1.25\times {{10}^{-1}}rad/s\]
done
clear
D)
\[1.56\,\,rad/\sec \]
done
clear
View Answer play_arrow
question_answer 14) Mass of moon is \[1/81\] times that of earth and its radius is \[1/4\] the radius of earth. If escape velocity on the surface of the earth is\[11.2\,\,km/s\]. Then the value of escape velocity at surface of the moon will be
A)
\[5\,\,km/s\]
done
clear
B)
\[2.5\,\,km/s\]
done
clear
C)
\[0.5\,\,km/\sec \]
done
clear
D)
\[0.14\,\,km/\sec \]
done
clear
View Answer play_arrow
question_answer 15) Pressure inside two soap bubbles are 1.01 and 1.02 atmospheres. Ratio between their volumes is
A)
\[2:1\]
done
clear
B)
\[8:1\]
done
clear
C)
\[108:101\]
done
clear
D)
\[{{(102)}^{2}}:{{(103)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 16) A bob of mass \[10\,\,kg\] is attached to wire \[0.3\] long. Its breaking stress is\[4.8\times {{10}^{7}}N{{m}^{2}}\]. The area of cross section of the wire is\[{{10}^{-6}}{{m}^{2}}\]. The maximum angular velocity with which it can be rotated in a horizontal circle
A)
\[8\,\,rad/\sec \]
done
clear
B)
\[4\,\,rad/\sec \]
done
clear
C)
\[2\,\,rad/\sec \]
done
clear
D)
\[1\,\,rad/\sec \]
done
clear
View Answer play_arrow
question_answer 17) A body cools from \[{{60}^{o}}C\] to \[{{50}^{o}}C\] in 10 minutes. If the room temperature is \[{{25}^{o}}C\] and assuming Newtons cooling law holds good, the temperature of the body at the end of next 10 minute is
A)
\[{{45}^{o}}C\]
done
clear
B)
\[{{42.85}^{o}}C\]
done
clear
C)
\[{{40}^{o}}C\]
done
clear
D)
\[{{38.5}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 18) The original temperature of a black body is\[{{727}^{o}}C\]. The temperature at which this black body must be raised so as to double, the total radiant energy, is
A)
\[971\,\,K\]
done
clear
B)
\[1190\,\,K\]
done
clear
C)
\[2001\,\,K\]
done
clear
D)
\[1458\,\,K\]
done
clear
View Answer play_arrow
question_answer 19) Molar specific heat at constant pressure for a mono atomic gas is
A)
\[\frac{8}{2}R\]
done
clear
B)
\[\frac{7}{2}R\]
done
clear
C)
\[\frac{5}{2}R\]
done
clear
D)
\[\frac{3}{2}R\]
done
clear
View Answer play_arrow
question_answer 20) A Carnot engine works between temperature\[{{727}^{o}}C\]and\[{{27}^{o}}C\]. The efficiency of the engine is
A)
\[70%\]
done
clear
B)
\[35%\]
done
clear
C)
\[90%\]
done
clear
D)
\[100%\]
done
clear
View Answer play_arrow
question_answer 21) A mono atomic gas \[\left( \gamma =\frac{5}{3} \right)\] is suddenly compressed to \[\frac{1}{8}th\] of its volume adiabatically. Then the pressure of the gas will change to
A)
\[\frac{47}{3}\]
done
clear
B)
\[18\]
done
clear
C)
\[\frac{29}{5}\]
done
clear
D)
32 times its initial pressure
done
clear
View Answer play_arrow
question_answer 22) One mole of an ideal mono atomic gas is heated at a constant pressure of one atmosphere from \[{{0}^{o}}C\] to\[{{100}^{o}}C\]. Then the change in the internal energy is
A)
\[3\times {{10}^{-4}}\,\,C\]
done
clear
B)
\[3\times {{10}^{-3}}\,\,C\]
done
clear
C)
\[3\times {{10}^{-2}}\,\,C\]
done
clear
D)
\[3\times {{10}^{-1}}\,\,C\]
done
clear
View Answer play_arrow
question_answer 23) When a mass \[m\] is attached to a spring, it normally extends by\[0.2\,\,m\]. The mass m is given a slight addition extension and released, then its time period will be
A)
\[\frac{1}{7}\]second
done
clear
B)
second
done
clear
C)
\[\frac{2\pi }{7}\]second
done
clear
D)
\[\frac{2}{3\pi }\]second
done
clear
View Answer play_arrow
question_answer 24) If the velocity of sound in air is\[350\,\,m/s\], then fundamental frequency of an open organ pipe of length \[50\,\,cm\] is
A)
\[980\,\,Hz\]
done
clear
B)
\[700\,\,Hz\]
done
clear
C)
\[350\,\,Hz\]
done
clear
D)
\[175\,\,Hz\]
done
clear
View Answer play_arrow
question_answer 25) A siren emitting sound of frequency \[800\,\,Hz\] is going away from a stationary listener with the speed of\[30\,\,m/s\]. The frequency of sound to be heard directly from the siren is
A)
\[733.3\,\,Hz\]
done
clear
B)
\[782.3\,\,Hz\]
done
clear
C)
\[833.3\,\,Hz\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 26) A particle executing SHM has amplitude \[0.01\] and frequency\[60\,\,Hz\] . The maximum acceleration of the particle is
A)
\[60\,\,{{\pi }^{2}}\,\,m/{{s}^{2}}\]
done
clear
B)
\[80\,\,{{\pi }^{2}}\,\,m/{{s}^{2}}\]
done
clear
C)
\[120\,\,{{\pi }^{2}}\,\,m/{{s}^{2}}\]
done
clear
D)
\[144\,\,{{\pi }^{2}}\,\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 27) If the equation of motion of a standing wave is \[y=0.3\sin (314t-1.57x)\]. Then the velocity of standing wave is
A)
\[400\,\,m/s\]
done
clear
B)
\[250\,\,m/s\]
done
clear
C)
\[100\,\,m/s\]
done
clear
D)
\[200\,\,m/s\]
done
clear
View Answer play_arrow
question_answer 28) The dielectric strength of air at \[NTP\] is \[3\times {{10}^{6}}\,\,V/m\] then the maximum charge that can be given to a spherical conductor of radius \[3\,\,m\] is
A)
\[3\times {{10}^{-4}}\,\,C\]
done
clear
B)
\[3\times {{10}^{-3}}\,\,C\]
done
clear
C)
\[3\times {{10}^{-2}}\,\,C\]
done
clear
D)
\[3\times {{10}^{-1}}\,\,C\]
done
clear
View Answer play_arrow
question_answer 29) In an hydrogen atom, the electron revolves around the nucleus in an orbit of radius\[0.53\times {{10}^{-10}}\,\,m\]. Then the electrical potential produced by nucleus at the position of the elect is
A)
\[-13.6\,\,V\]
done
clear
B)
\[-27.2\,\,V\]
done
clear
C)
\[27.2\,\,V\]
done
clear
D)
\[13.6\,\,V\]
done
clear
View Answer play_arrow
question_answer 30) Two spherical conductor of capacitances \[3\mu F\] and \[5\mu F\] are charged to potent of \[300\,\,volt\] and\[500\,\,volt\]. The two connected resulting in redistribution charges. The decrease in electrical energy will be
A)
zero
done
clear
B)
\[37.3\times {{10}^{-3}}\,\,\text{J}\]
done
clear
C)
\[722.5\times {{10}^{-3}}\]
done
clear
D)
\[760\times {{10}^{-3}}\,\,\text{J}\]
done
clear
View Answer play_arrow
question_answer 31) Three capacitors of \[2.0,\,\,3.0,\,\,6.0\,\,\mu F\] are connected in series to a \[10\,\,volt\] source. The charge on \[3.0\,\,\mu F\] capacitor is
A)
\[15\,\,\mu C\]
done
clear
B)
\[12\,\,\mu C\]
done
clear
C)
\[5\,\,\mu C\]
done
clear
D)
\[10\,\,\mu C\]
done
clear
View Answer play_arrow
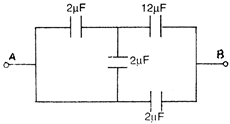
question_answer 32)
Four capacitors are connected in circuit as shown in the given figure. The effective capacitance between points \[A\] and \[B\] will be
A)
\[4\,\,\mu F\]
done
clear
B)
\[18\,\,\mu F\]
done
clear
C)
\[\frac{28}{9}\,\,\mu F\]
done
clear
D)
\[5\,\,\mu F\]
done
clear
View Answer play_arrow
question_answer 33) From a point charge, there is a fix point\[A\]. At\[A\], there is an electric fit of \[500\,\,V/m\] and potential difference\[3000\,\,V\]. Distance between point char and point \[A\] is
A)
\[24\,\,m\]
done
clear
B)
\[16\,\,m\]
done
clear
C)
\[12\,\,m\]
done
clear
D)
\[6\,\,m\]
done
clear
View Answer play_arrow
question_answer 34) There is a current of \[40\] ampere in a wire of \[{{10}^{-6}}{{m}^{2}}\] area of cross-section. If the number of free electron per \[{{m}^{3}}\] is\[{{10}^{29}}\], then the drift velocity will be
A)
\[1.25\times {{10}^{3}}\,\,m/s\]
done
clear
B)
\[2.50\times {{10}^{-3}}\,\,m/s\]
done
clear
C)
\[25.0\times {{10}^{-3}}\,\,m/s\]
done
clear
D)
\[250\times {{10}^{-3}}\,\,m/s\]
done
clear
View Answer play_arrow
question_answer 35) A \[50\,\,volt\] battery is connected across a 10 ohm resistor. The current is 4.5 amp. The internal resistance of battery will be
A)
\[5.0\,\,\Omega \]
done
clear
B)
\[1.1\,\,\Omega \]
done
clear
C)
\[0.5\,\,\Omega \]
done
clear
D)
\[zero\].
done
clear
View Answer play_arrow
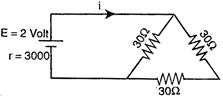
question_answer 36)
The current i in the circuit of the adjoining fig is
A)
\[\frac{1}{5}\,\,amp\]
done
clear
B)
\[\frac{1}{10}\,\,amp\]
done
clear
C)
\[\frac{1}{45}\,\,amp\]
done
clear
D)
\[\frac{1}{15}\,\,amp\]
done
clear
View Answer play_arrow
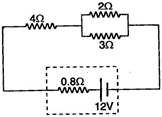
question_answer 37)
A battery of \[12\,\,V\] and an internal resistance of \[0.80\] is connected to 3 resistors as shown in the fig. The current ( in the circuit is
A)
\[2.3\,\,A\]
done
clear
B)
\[4.0\,\,A\]
done
clear
C)
\[2.0\,\,A\]
done
clear
D)
\[1.33\,\,A\]
done
clear
View Answer play_arrow
question_answer 38) A prism of refracting angle \[{{60}^{o}}\] has minimum angle of deviation of\[{{30}^{o}}\]. What must be the angle of incidence for this case?
A)
\[{{30}^{o}}\]
done
clear
B)
\[{{45}^{o}}\]
done
clear
C)
\[{{90}^{o}}\]
done
clear
D)
\[{{0}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 39) Time taken by light to cross a glass slab of thickness 4 mm and refractive index 3, is
A)
\[4\times {{10}^{-11}}s\]
done
clear
B)
\[2\times {{10}^{-11}}s\]
done
clear
C)
\[16\times {{10}^{-11}}s\]
done
clear
D)
\[8\times {{10}^{-11}}s\]
done
clear
View Answer play_arrow
question_answer 40) Youngs double slit experiment is performed with light of wavelength\[550\,\,nm\]. The separation between the slits is \[1.10\,\,mm\] and screen is placed at distance of\[1\,\,m\]. What is the distance between the consecutive bright and dark fringes?
A)
\[1.5\,\,mm\]
done
clear
B)
\[1.0\,\,mm\]
done
clear
C)
\[0.5\,\,mm\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 41) What will be the angle of diffracting for the first minimum due to Fraun hoffer diffraction with sources of light of wave length \[550\,\,nm\] and slit of width\[0.55\,\,mm\]
A)
\[0.001\,\,rad\]
done
clear
B)
\[0.01\,\,rad\]
done
clear
C)
\[1\,\,rad\]
done
clear
D)
\[0.1\,\,rad\]
done
clear
View Answer play_arrow
question_answer 42) Magnifying power of an astronomical telescope is \[8\] and the distance between the two lenses is\[54\,\,cm\]. The focal length of eye lens and objective lens will be respectively.
A)
\[64cm\And 8cm\]
done
clear
B)
\[8cm\And 64cm\]
done
clear
C)
\[48cm\And 6cm\]
done
clear
D)
\[6cm\And 48cm\]
done
clear
View Answer play_arrow
question_answer 43) An electron jumps from 5th orbit to 4th orbit of hydrogen atom. Taking the Rydberg constant as \[{{10}^{7}}\] per minute. What will be the frequency of radiation emitted?
A)
\[6.75\times {{10}^{12}}Hz\]
done
clear
B)
\[6.75\times {{10}^{14}}Hz\]
done
clear
C)
\[6.75\times {{10}^{13}}Hz\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 44) Ultraviolet radiation of \[6.2\,\,eV\] falls on an aluminium surface (work function\[4.2\,\,eV\]). The kinetic energy in joule of the fastest electron emitted is approximately
A)
\[3\times {{10}^{-15}}\,\,J\]
done
clear
B)
\[4\times {{10}^{-17}}\,\,J\]
done
clear
C)
\[3\times {{10}^{-19}}\,\,J\]
done
clear
D)
\[3\times {{10}^{-21}}\,\,J\]
done
clear
View Answer play_arrow
question_answer 45) The binding energy of deuterium and helium atom is \[1.1\,\,MeV\] and\[7.0\,\,MeV\]. If two deuterium nuclei fuse to form helium atom, the energy released is
A)
\[19.2\,\,MeV\]
done
clear
B)
\[23.6\,\,MeV\]
done
clear
C)
\[26.9\,\,MeV\]
done
clear
D)
\[13.9\,\,MeV\]
done
clear
View Answer play_arrow
question_answer 46) \[Bi\] has a half-life of 5 days. What time is taken by \[(7/8)th\] part of the sample to decay?
A)
20 day
done
clear
B)
15 day
done
clear
C)
10 day
done
clear
D)
3-4 day
done
clear
View Answer play_arrow
question_answer 47) The impurity atoms with which a pure silicon should be doped to make a\[p-\]type semiconductor is
A)
phosphorous
done
clear
B)
aluminium
done
clear
C)
antimony
done
clear
D)
arsenic
done
clear
View Answer play_arrow
question_answer 48) A transistor has an \[\alpha =0.95\] it has charge in emitter current of \[100\,\,milli\] ampere, then the charge in collector current is
A)
\[95\,\,mA\]
done
clear
B)
\[99.05\,\,mA\]
done
clear
C)
\[00.95\,\,mA\]
done
clear
D)
\[100\,\,mA\]
done
clear
View Answer play_arrow
question_answer 49) A coil of resistance \[10\Omega \] and an inductance \[5\,\,H\] is connected to a \[100\,\,volt\] battery. Then energy stored in the coil is
A)
\[125\,\,erg\]
done
clear
B)
\[125\,\,J\]
done
clear
C)
\[250\,\,erg\]
done
clear
D)
\[250\,\,J\]
done
clear
View Answer play_arrow
question_answer 50) The velocity of electromagnetic waves in a good conductor is
A)
\[3\times {{10}^{8}}\,\,m/s\]
done
clear
B)
zero
done
clear
C)
infinite
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 51) Ethylene is obtained from ethyl bromide by treating it with
A)
hydrogen
done
clear
B)
aqueous caustic soda
done
clear
C)
aqueous caustic potash
done
clear
D)
alcoholic caustic potash
done
clear
View Answer play_arrow
question_answer 52) Which substance when boiled with \[NaOH\] will evolve\[N{{H}_{3}}\]?
A)
Aniline
done
clear
B)
Acetamide
done
clear
C)
Ethylamine
done
clear
D)
Acetoxime
done
clear
View Answer play_arrow
question_answer 53) Optical isomerism shown by
A)
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}OH\]
done
clear
B)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{2}}-OH\]
done
clear
C)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
D)
\[CH-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-OH\]
done
clear
View Answer play_arrow
question_answer 54) Length of carbon-carbon bond is least in
A)
ethanol
done
clear
B)
ethanol
done
clear
C)
ethane
done
clear
D)
ethyne
done
clear
View Answer play_arrow
question_answer 55) Raffinose is
A)
trisaccharide
done
clear
B)
monosaccharide
done
clear
C)
disaccharide
done
clear
D)
none
done
clear
View Answer play_arrow
question_answer 56) Formaldehyde on condensation in presence of \[Ca{{(OH)}_{2}}\] gives
A)
formose
done
clear
B)
fructose
done
clear
C)
maltose
done
clear
D)
xylose
done
clear
View Answer play_arrow
question_answer 57) The reaction between chlorobenzene and chloral in the presence of concentrated sulphuric acid produces
A)
gammexane
done
clear
B)
\[p.p-\]dichloro diphenyl trichloro ethane
done
clear
C)
chloropicrin
done
clear
D)
benzene hexachloride
done
clear
View Answer play_arrow
question_answer 58) Which does not react with Fehlings solution?
A)
Acetaldehyde
done
clear
B)
Formic acid
done
clear
C)
Glucose
done
clear
D)
Benzaldehyde
done
clear
View Answer play_arrow
question_answer 59) Primary alcohols can be obtained from the reaction of the \[RMgX\] with
A)
\[C{{O}_{2}}\]
done
clear
B)
\[HCHO\]
done
clear
C)
\[C{{H}_{3}}CHO\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 60) \[PC{{l}_{5}}\] reacts with propanone, to give
A)
vic-dichloride
done
clear
B)
propanal
done
clear
C)
propane-chloride
done
clear
D)
gem-dichloride
done
clear
View Answer play_arrow
question_answer 61) Which is a suitable reagent to confirm the presence of iodine?
A)
Fehlings solution
done
clear
B)
Starch solution
done
clear
C)
Silver nitrate solution
done
clear
D)
Miltons reagent
done
clear
View Answer play_arrow
question_answer 62) Concentrated \[NaOH\] and benzaldehyde react to produce
A)
cinnamic acid
done
clear
B)
hydrogen benzamide
done
clear
C)
benzophenone
done
clear
D)
benzyl alcohol
done
clear
View Answer play_arrow
question_answer 63) When wine is put in air, it becomes sour due to
A)
reduction of\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
B)
dissolution of\[C{{O}_{2}}\]
done
clear
C)
oxidation of\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
D)
formation of\[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 64) Which radicals are precipitated in \[{{(N{{H}_{4}})}_{2}}C{{O}_{3}}\] in presence of alkali?
A)
\[C{{a}^{+2}},\,\,B{{a}^{+2}},\,\,S{{r}^{+2}}\]
done
clear
B)
\[Mg\]
done
clear
C)
Both
done
clear
D)
None
done
clear
View Answer play_arrow
question_answer 65) At what temperature will be rate of effusion of \[{{N}_{2}}\] be \[1.625\] time to rate of effusion of \[S{{O}_{2}}\] at\[{{500}^{o}}C\]?
A)
\[1730\,\,K\]
done
clear
B)
\[110\,\,K\]
done
clear
C)
\[273\,\,K\]
done
clear
D)
\[893\,\,K\]
done
clear
View Answer play_arrow
question_answer 66) Oxygen contains \[90%\,\,{{O}^{16}}\] and\[10%\]\[{{O}^{18}}\]. Its atomic mass is
A)
\[16.2\]
done
clear
B)
\[17.4\]
done
clear
C)
\[17\]
done
clear
D)
\[16.5\]
done
clear
View Answer play_arrow
question_answer 67) White smoke is formed when ammonia gas meets with
A)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[HCl\]
done
clear
C)
\[{{H}_{2}}O\]
done
clear
D)
\[HN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 68) According to Bronsted-Lowry concept, the correct order of relative strength of bases follows the order
A)
\[C{{H}_{3}}CO{{O}^{-}}>C{{l}^{-}}>O{{H}^{-}}\]
done
clear
B)
\[C{{H}_{3}}CO{{O}^{-}}>O{{H}^{-}}>C{{l}^{-}}\]
done
clear
C)
\[O{{H}^{-}}>C{{H}_{3}}CO{{O}^{-}}>C{{l}^{-}}\]
done
clear
D)
\[O{{H}^{-}}>C{{l}^{-}}>C{{H}_{3}}CO{{O}^{-}}\]
done
clear
View Answer play_arrow
question_answer 69) The common ion effect is shown by which of the following set of solutions?
A)
\[BaC{{l}_{2}}+BaN{{O}_{3}}\]
done
clear
B)
\[NaCl+HCl\]
done
clear
C)
\[N{{H}_{4}}OH+N{{H}_{4}}Cl\]
done
clear
D)
None
done
clear
View Answer play_arrow
question_answer 70) The number of incomplete orbitals in inner transition elements are
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 71) What is \[\Delta n\] for combustion of 1 mole of benzene, when both the reactants and the products are gas at\[298\,\,K\]
A)
\[0\]
done
clear
B)
\[3/2\]
done
clear
C)
\[-3/2\]
done
clear
D)
\[1/2\]
done
clear
View Answer play_arrow
question_answer 72) Tincal is
A)
\[N{{a}_{2}}C{{O}_{3}}.10{{H}_{2}}O\]
done
clear
B)
\[NaN{{O}_{3}}\]
done
clear
C)
\[NaCl\]
done
clear
D)
\[N{{a}_{2}}{{B}_{4}}{{O}_{7}}.10{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 73) lonisation potential values of \[d\] block elements as compared to ionization potential value of \[f\] block elements are
A)
higher
done
clear
B)
equal
done
clear
C)
lower
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 74) The distribution law is applied for the distribution of basic acid between
A)
water and ethyl alcohol
done
clear
B)
water and amyl alcohol
done
clear
C)
water and sulphuric acid
done
clear
D)
water and liquor ammonia
done
clear
View Answer play_arrow
question_answer 75) The maximum sum of the number of neutrons and protons in an isotope of hydrogen is
A)
4
done
clear
B)
5
done
clear
C)
6
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 76) The amount of \[{{H}_{2}}S{{O}_{4}}\] present in \[400\,\,ml\] of \[0.1\,\,M\]solution is
A)
\[4.9\,\,g\]
done
clear
B)
\[9.80\,\,g\]
done
clear
C)
\[3.92\,\,g\]
done
clear
D)
\[12.45\,\,g\]
done
clear
View Answer play_arrow
question_answer 77) The compound which ionizes almost completely in water is
A)
alcohol
done
clear
B)
acetic acid
done
clear
C)
hydrogen peroxide
done
clear
D)
potassium chloride
done
clear
View Answer play_arrow
question_answer 78) On addition of an inert gas at constant volume to the reaction\[{{N}_{2}}+3{{H}_{2}}2N{{H}_{3}}\]at equilibrium
A)
the reaction remains unaffected
done
clear
B)
forward reaction is favoured
done
clear
C)
the reaction halts
done
clear
D)
backward reaction is favoured
done
clear
View Answer play_arrow
question_answer 79) Which shows highest coagulating power for negatively charged \[A{{g}_{2}}{{S}_{3}}\] colloid solution?
A)
\[SO_{2}^{4-}\]
done
clear
B)
\[PO_{4}^{3-}\]
done
clear
C)
\[A{{l}^{3+}}\]
done
clear
D)
\[N{{a}^{+}}\]
done
clear
View Answer play_arrow
question_answer 80) Temporary hardness of water can be removed by
A)
addition of potassium permagenate
done
clear
B)
boiling
done
clear
C)
filtration
done
clear
D)
addition of chlorine
done
clear
View Answer play_arrow
question_answer 81) Heat or formation of \[{{H}_{2}}O(g)\] at \[1\,\,atm\] and \[{{25}^{o}}C\] is\[-243\,\,kJ\]. \[\Delta E\] for the reaction \[{{H}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)\to {{H}_{2}}O(g)\]at \[{{25}^{o}}C\] is
A)
\[-243\,\,kJ\]
done
clear
B)
\[-241.8\,\,kJ\]
done
clear
C)
\[241.8\,\,kJ\]
done
clear
D)
\[243\,\,kJ\]
done
clear
View Answer play_arrow
question_answer 82) In the reduction of dichromate by \[Fe(II)\] , the number of electrons involved per chromium atom is
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 83) \[0.71\,\,g\]of chlorine combines with certain weight of a metal giving \[1.11\,\,g\] of its chloride. The equivalent weight of the metal is
A)
\[40\]
done
clear
B)
\[20\]
done
clear
C)
\[80\]
done
clear
D)
none
done
clear
View Answer play_arrow
question_answer 84) In the following reaction,\[2S{{O}_{2}}(g)+{{O}_{2}}(g)\to 2S{{O}_{3}}(g)\]:\[\Delta H=-\]heat, the formation of \[S{{O}_{3}}\] is favoured by
A)
decreasing the pressure and increasing the temperature
done
clear
B)
increasing the pressure and decreasing the temperature
done
clear
C)
increasing the pressure and the temperature
done
clear
D)
decreasing the pressure and the temperature
done
clear
View Answer play_arrow
question_answer 85) A metal oxide is reduced by heating it in a stream of hydrogen. It is found that after complete reduction, \[3.15\,\,g\] of the oxide have yielded \[1.05\] of the metal. We may deduce that
A)
the eq. weight of the metal is 8
done
clear
B)
the atomic weight of the metal is 8
done
clear
C)
the atomic weight of the metal is 4
done
clear
D)
the eq. weight of the metal is 4.
done
clear
View Answer play_arrow
question_answer 86) An electron is in one of the 3 \[d-\]orbitals, which of the quantum number is not possible?
A)
\[n=1\]
done
clear
B)
\[n=3\]
done
clear
C)
\[l=1\]
done
clear
D)
\[m=2\]
done
clear
View Answer play_arrow
question_answer 87) The \[pH\] of \[1%\] ionised \[0.1\,\,M\] solution of a weak monoprotic acid is
A)
\[3\]
done
clear
B)
\[1\]
done
clear
C)
\[2\]
done
clear
D)
\[11\]
done
clear
View Answer play_arrow
question_answer 88) A system is changed from state \[A\] to state \[B\] by one path and from \[B\] to \[A\] another path. If\[{{E}_{1}}\] and \[{{E}_{2}}\] are the corresponding changes in internal energy, then
A)
\[{{E}_{1}}+{{E}_{2}}=-ve\]
done
clear
B)
\[{{E}_{1}}+{{E}_{2}}=+ve\]
done
clear
C)
\[{{E}_{1}}+{{E}_{2}}=0\]
done
clear
D)
none
done
clear
View Answer play_arrow
question_answer 89) Suppose you have to determine the percentage of carbon dioxide in a sample of a gas available in a container. Which is the best absorbing material for the carbon dioxide is
A)
Heated copper oxide
done
clear
B)
Cold, solid calcium chloride
done
clear
C)
Cold, solid calcium hydroxide
done
clear
D)
Heated charcoal
done
clear
View Answer play_arrow
question_answer 90) Which compound has bond angle nearly to 90?
A)
\[{{H}_{2}}O\]
done
clear
B)
\[{{H}_{2}}S\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[C{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 91) The bond angle is minimum in
A)
\[{{H}_{2}}Te\]
done
clear
B)
\[{{H}_{2}}Se\]
done
clear
C)
\[{{H}_{2}}O\]
done
clear
D)
\[{{H}_{2}}S\]
done
clear
View Answer play_arrow
question_answer 92) If two light nuclei are fused together in nuclear reaction the average energy per nucleon
A)
increases
done
clear
B)
cannot be determined
done
clear
C)
remains same
done
clear
D)
decreases
done
clear
View Answer play_arrow
question_answer 93) Which metal does not give the following reaction? \[M+water\,\,or\,\,stream\xrightarrow{{}}oxide\,\,+{{H}_{2}}\uparrow \]
A)
Mercury
done
clear
B)
Iron
done
clear
C)
Sodium
done
clear
D)
Magnesium
done
clear
View Answer play_arrow
question_answer 94) What bond order does\[O_{2}^{2-}\]have
A)
3
done
clear
B)
2
done
clear
C)
1
done
clear
D)
½
done
clear
View Answer play_arrow
question_answer 95) Which noble gas is the least polarizable?
A)
\[Kr\]
done
clear
B)
\[Ne\]
done
clear
C)
\[He\]
done
clear
D)
\[Rn\]
done
clear
View Answer play_arrow
question_answer 96) Which one is the strongest bond?
A)
\[Br-F\]
done
clear
B)
\[F-F\]
done
clear
C)
\[Cl-F\]
done
clear
D)
\[Br-Cl\]
done
clear
View Answer play_arrow
question_answer 97) The oxidation number of \[N\] in \[{{N}_{2}}H_{5}^{+}\] is
A)
\[-3\]
done
clear
B)
\[(-2)\]
done
clear
C)
\[-1\]
done
clear
D)
\[+2\]
done
clear
View Answer play_arrow
question_answer 98) Aquaregia is a mixture of
A)
\[{{H}_{3}}P{{O}_{4}}+{{H}_{2}}S{{O}_{4}}\]
done
clear
B)
\[3HN{{O}_{3}}+HCl\]
done
clear
C)
\[3HCl+HN{{O}_{3}}\]
done
clear
D)
\[HCl+C{{H}_{3}}COOH\]
done
clear
View Answer play_arrow
question_answer 99) The electronic configuration of halogen is
A)
\[n{{s}^{2}}n{{p}^{6}}\]
done
clear
B)
\[n{{s}^{2}}n{{p}^{3}}\]
done
clear
C)
\[n{{s}^{2}}n{{p}^{5}}\]
done
clear
D)
\[n{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 100) On warming formic acid with ammonical silver nitrate, the product formed is
A)
silver formate
done
clear
B)
metallic silver
done
clear
C)
silver oxide
done
clear
D)
formaldehyde
done
clear
View Answer play_arrow
question_answer 101) Which of the following structure is present in mitochondria?
A)
Polysomes
done
clear
B)
Dilysomes
done
clear
C)
Quantasomes
done
clear
D)
Oxysomes
done
clear
View Answer play_arrow
question_answer 102) Cell theory was propounded by
A)
Schleiden and Schwann
done
clear
B)
Robert Hooke
done
clear
C)
Robert Koch
done
clear
D)
Darwin
done
clear
View Answer play_arrow
question_answer 103) Which process is completed in mitochondria?
A)
Oxidative phosphorylation
done
clear
B)
Photophosphorylation
done
clear
C)
Photolysis
done
clear
D)
Matrix formation
done
clear
View Answer play_arrow
question_answer 104) Grana are present inside the
A)
mitochondria
done
clear
B)
chloroplast
done
clear
C)
endoplasmic reticulum
done
clear
D)
ribosome
done
clear
View Answer play_arrow
question_answer 105) Which event involves in crossing over?
A)
Chromosomes are short and thick
done
clear
B)
Exchange of genetic material
done
clear
C)
Pairing of chromosomes
done
clear
D)
Addition of chromosomes
done
clear
View Answer play_arrow
question_answer 106) Insectivorous plants grow in
A)
calcium deficient soil
done
clear
B)
carbon deficient soil
done
clear
C)
magnesium deficient soil
done
clear
D)
nitrogen deficient soil
done
clear
View Answer play_arrow
question_answer 107) The term water potential was given by
A)
Dixon and Jolly
done
clear
B)
Prof. R.C. Sirohi
done
clear
C)
J.C. Bose
done
clear
D)
Slatyer and Tayler
done
clear
View Answer play_arrow
question_answer 108) Abscisic acid plays active role in
A)
dormancy of seeds
done
clear
B)
cell division
done
clear
C)
enhance ,senscense
done
clear
D)
shoot elongation
done
clear
View Answer play_arrow
question_answer 109) What is R. Q. of early stages of germination of castor seed?
A)
One
done
clear
B)
Two
done
clear
C)
Zero
done
clear
D)
Less than one
done
clear
View Answer play_arrow
question_answer 110) The female gametophyte is made up of
A)
single celled
done
clear
B)
six celled
done
clear
C)
seven celled
done
clear
D)
eight celled
done
clear
View Answer play_arrow
question_answer 111) Humus is
A)
living organic and inorganic matter
done
clear
B)
radioactive materials
done
clear
C)
dead and decayed organic matter
done
clear
D)
only living organic matter
done
clear
View Answer play_arrow
question_answer 112) In nitrogen deficient soil, which group of plant can grow?
A)
Gymnosperms
done
clear
B)
Algae
done
clear
C)
Insectivorous
done
clear
D)
Bryophytes
done
clear
View Answer play_arrow
question_answer 113) Which family has actinomorphic flower?
A)
Poaceae
done
clear
B)
Solanaceae
done
clear
C)
Papaveraceae
done
clear
D)
Papilionaceae
done
clear
View Answer play_arrow
question_answer 114) Classification given by Hutchinson is
A)
phylogenetic
done
clear
B)
artificial
done
clear
C)
natural
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 115) The depletion of ozone layer in stratosphere would result in
A)
global warming
done
clear
B)
forest fires
done
clear
C)
skin cancer incidence
done
clear
D)
soil erosion
done
clear
View Answer play_arrow
question_answer 116) Crop rotation is used by farmers
A)
to increases soil fertility
done
clear
B)
to increases community area
done
clear
C)
to increases organic content of the soil
done
clear
D)
to increases nitrogenous content in the soil
done
clear
View Answer play_arrow
question_answer 117) Soil erosion can be prevented by
A)
afforestation
done
clear
B)
deforestation
done
clear
C)
over-grazing
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 118) Opium is obtained from which plant
A)
Tectona grandis
done
clear
B)
Triticum aestivum
done
clear
C)
Papaver somniferum
done
clear
D)
Rosa indica
done
clear
View Answer play_arrow
question_answer 119) Which is not a topographic factor?
A)
Altitude
done
clear
B)
Steepness of slope
done
clear
C)
Direction of slope
done
clear
D)
Wind velocity
done
clear
View Answer play_arrow
question_answer 120) Latex yielding plant is
A)
Simmondria chinensis
done
clear
B)
Parthenium argentatum
done
clear
C)
Triticum aestivum
done
clear
D)
Mangifera indica
done
clear
View Answer play_arrow
question_answer 121) Main effect of \[S{{O}_{2}}\] on plants is
A)
golgi complex distruction
done
clear
B)
chlorophyll distruction
done
clear
C)
deplasmolysis
done
clear
D)
plamolysis
done
clear
View Answer play_arrow
question_answer 122) In leguminous plants, which pigment is essential for \[{{N}_{3}}\] fixation?
A)
Xanthophyll
done
clear
B)
Phycocyanin
done
clear
C)
Leghaemoglobin
done
clear
D)
Anthocyanin
done
clear
View Answer play_arrow
question_answer 123) In phototropism, which hormone is involved
A)
\[2,\,\,4,\,\,5-T\]
done
clear
B)
\[2,\,\,4-D\]
done
clear
C)
cytokinin
done
clear
D)
\[IAA\]
done
clear
View Answer play_arrow
question_answer 124) Which bacteria is associated with roots of legume plants?
A)
Rhizobium
done
clear
B)
Nostoc
done
clear
C)
Spirogyra
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 125) Protoplasmic streaming theory was first proposed by
A)
J. C. Bose
done
clear
B)
Dixon and Jolly
done
clear
C)
de Vries
done
clear
D)
Curtis
done
clear
View Answer play_arrow
question_answer 126) Which is used in culture medium?
A)
Agar-Agar
done
clear
B)
Resin
done
clear
C)
Gum
done
clear
D)
Paint
done
clear
View Answer play_arrow
question_answer 127) In Ficus inflorescence is
A)
hypanthodium
done
clear
B)
cymose
done
clear
C)
raceme
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 128) Which shows heterothallism?
A)
Ephedra
done
clear
B)
Pinus
done
clear
C)
Rhizopus
done
clear
D)
Cycas
done
clear
View Answer play_arrow
question_answer 129) Peter Mitchell is related with
A)
Photosynthetic theory
done
clear
B)
Chemiosmotic theory
done
clear
C)
Virology
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 130) Which type of pollution is checked by green mufler?
A)
Noise
done
clear
B)
Soil
done
clear
C)
Water
done
clear
D)
Air
done
clear
View Answer play_arrow
question_answer 131) The disease minimata is produced by the pollution of water with
A)
calcium chloride
done
clear
B)
silver nitrate
done
clear
C)
mercuric chloride
done
clear
D)
aluminium
done
clear
View Answer play_arrow
question_answer 132) Major function of roots growing in air of marshy plants growing in saline soil is
A)
absorption of nutrients
done
clear
B)
absorb moisture
done
clear
C)
salt injury
done
clear
D)
respire
done
clear
View Answer play_arrow
question_answer 133) In plants healing of wounds takes place by the activity of
A)
intercalary meristem
done
clear
B)
apical meristem
done
clear
C)
lateral meristem
done
clear
D)
secondary meristem
done
clear
View Answer play_arrow
question_answer 134) Characteristic of legumes is the assimilation of \[{{N}_{2}}\] in the form of
A)
direct\[{{N}_{3}}\]
done
clear
B)
chloride
done
clear
C)
nitrates
done
clear
D)
ammonia
done
clear
View Answer play_arrow
question_answer 135) Dicot leaf showing parallel venation belongs to the genus
A)
Calophyllum
done
clear
B)
Zea
done
clear
C)
Triticum
done
clear
D)
Pisum
done
clear
View Answer play_arrow
question_answer 136) In horticulture, which chemical is used as root inducing
A)
Ethylene
done
clear
B)
ATP
done
clear
C)
ABA
done
clear
D)
IBA
done
clear
View Answer play_arrow
question_answer 137) Botulism is caused by Clostridium botiilinum this bacteria is
A)
facultative anaerobes
done
clear
B)
obligate anaerobes
done
clear
C)
facultative aerobes
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 138) Chloroplasts, with pyrenoid are found in the leaves of
A)
Funaria
done
clear
B)
wheat
done
clear
C)
rice
done
clear
D)
maize
done
clear
View Answer play_arrow
question_answer 139) Which enzyme is electron transfer enzyme?
A)
Lypases
done
clear
B)
Proteases
done
clear
C)
Cytochromes
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 140) In pyramid of fresh water pond ecosystem number of primary consumers is
A)
more than the secondary consumers
done
clear
B)
equal to producers
done
clear
C)
less than the secondary consumers
done
clear
D)
more than the producers
done
clear
View Answer play_arrow
question_answer 141) A- stalk-like structure which is found above the stamens and below the ovary is called
A)
Filament
done
clear
B)
gynophore
done
clear
C)
abdophore
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 142) Edaphology is a relationship between
A)
plant and ecosystem
done
clear
B)
plant and biosphere
done
clear
C)
soil and living organisms
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 143) In Cycus stem contains
A)
heart wood
done
clear
B)
sapwood
done
clear
C)
pycnoxylic wood
done
clear
D)
manoxylic wood
done
clear
View Answer play_arrow
question_answer 144) Which is edible in banana?
A)
Mesocarp
done
clear
B)
Endocarp
done
clear
C)
both \[a\] and \[b\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 145) In the same plant if pollen of a flower falls on the stigma of another flower
A)
cleistogamy
done
clear
B)
geitonogamy
done
clear
C)
both a and b
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 146) Which is closely associated with the energy conversion reactions of aerobic respiration?
A)
Mitochondria
done
clear
B)
Chloroplast
done
clear
C)
Golgi body
done
clear
D)
Nucleus
done
clear
View Answer play_arrow
question_answer 147) In Dionea the movement of lamina lobe is
A)
seismonastic
done
clear
B)
thigmonastic
done
clear
C)
photonastic
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 148) In plants auxin synthesis occurs in
A)
xylem cells
done
clear
B)
epidermis
done
clear
C)
root and shoot tips
done
clear
D)
phloem cells
done
clear
View Answer play_arrow
question_answer 149) In pond ecosystem diatoms represent
A)
producers
done
clear
B)
consumers
done
clear
C)
both \[a\] and \[b\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 150) More elongation of plant stem is due to
A)
cytokinin
done
clear
B)
ethylene
done
clear
C)
auxin
done
clear
D)
gibberellin
done
clear
View Answer play_arrow
question_answer 151) Lacteals are found in
A)
villus of intestine
done
clear
B)
kidneys
done
clear
C)
liver
done
clear
D)
lungs
done
clear
View Answer play_arrow
question_answer 152) Which one of the following exhibit concentric tube within tube plan?
A)
Echinodermata
done
clear
B)
Mollusca
done
clear
C)
Oligochaeta
done
clear
D)
Arthropoda
done
clear
View Answer play_arrow
question_answer 153) Vocal cords are situated at
A)
bronchial tube
done
clear
B)
glottis
done
clear
C)
larynx
done
clear
D)
pharynx
done
clear
View Answer play_arrow
question_answer 154) Karyotaxonomy is the modern branch of classification which is based on
A)
trinomial nomenclature
done
clear
B)
organic evolution
done
clear
C)
bands found on chromosomes
done
clear
D)
number of chromosomes
done
clear
View Answer play_arrow
question_answer 155) Under certain conditions scientist have obtained cell like structures. These are known as
A)
prebiotic soup
done
clear
B)
coacervates
done
clear
C)
protists
done
clear
D)
microbes
done
clear
View Answer play_arrow
question_answer 156) What is the name of the book written by Aristotle?
A)
Philosophic Zoologique
done
clear
B)
Systema Naturae
done
clear
C)
Histoire Naturelle
done
clear
D)
Historia Animalium
done
clear
View Answer play_arrow
question_answer 157) Which one of the following was most likely absent in free form in the primordial atmosphere at the time of origin of life?
A)
Oxygen
done
clear
B)
Ammonia
done
clear
C)
Hydrogen
done
clear
D)
Methane
done
clear
View Answer play_arrow
question_answer 158) In which book has Binomial Nomenclature been used for the first time?
A)
Historia Plantarum
done
clear
B)
Historia Naturalis
done
clear
C)
Systema Naturae
done
clear
D)
Histoire Naturelle
done
clear
View Answer play_arrow
question_answer 159) Haemoglobin is having maximum affinity with
A)
\[N{{H}_{3}}\]
done
clear
B)
\[{{O}_{2}}\]
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
\[CO\]
done
clear
View Answer play_arrow
question_answer 160) In mongolism a patient possesses
A)
44 chromosomes
done
clear
B)
45 chromosomes
done
clear
C)
47 chromosomes
done
clear
D)
46 chromosomes
done
clear
View Answer play_arrow
question_answer 161) Which blood group is a universal recipient?
A)
\[O\]
done
clear
B)
\[AB\]
done
clear
C)
\[B\]
done
clear
D)
\[A\]
done
clear
View Answer play_arrow
question_answer 162) When released from ovary, the human egg contains
A)
\[XY\] chromosomes
done
clear
B)
two \[X\] chromosomes
done
clear
C)
one \[Y\] chromosomes
done
clear
D)
one \[X\] chromosomes
done
clear
View Answer play_arrow
question_answer 163) In which blood group antigens are absent?
A)
\[O\]
done
clear
B)
\[B\]
done
clear
C)
\[AB\]
done
clear
D)
\[A\]
done
clear
View Answer play_arrow
question_answer 164) Which endocrine gland is responsible for immunity?
A)
Adrenal
done
clear
B)
Pituitary
done
clear
C)
Thymus
done
clear
D)
Pineal
done
clear
View Answer play_arrow
question_answer 165) Restriction endonuclease is used in
A)
regeneration of tissues
done
clear
B)
cell fractionation
done
clear
C)
tissue culture
done
clear
D)
genetic engineering
done
clear
View Answer play_arrow
question_answer 166) The function of amniotic cavity is
A)
protection from shocks
done
clear
B)
protection from dessication
done
clear
C)
protection from dessication and shocks
done
clear
D)
respiration
done
clear
View Answer play_arrow
question_answer 167) Full form of AIDS is
A)
acquired immune disease symptom
done
clear
B)
acquired immune deficiency syndrome
done
clear
C)
auto immune deficiency syndrome
done
clear
D)
anti-immune deficiency syndrome
done
clear
View Answer play_arrow
question_answer 168) Incomplete division of egg during cleavage is known as
A)
meroblastic
done
clear
B)
holoblastic
done
clear
C)
meridional
done
clear
D)
spiral
done
clear
View Answer play_arrow
question_answer 169) The process by which ova are formed is known as?
A)
Oviparity
done
clear
B)
Oviposition
done
clear
C)
Ovulation
done
clear
D)
Oogenesis
done
clear
View Answer play_arrow
question_answer 170) Cell division in sexual reproduction is
A)
meiotic
done
clear
B)
mitotic
done
clear
C)
amitotic
done
clear
D)
both \[a\] and \[b\]
done
clear
View Answer play_arrow
question_answer 171) The disease rickets of bones is due to
A)
vitamin D deficiency
done
clear
B)
vitamin C deficiency
done
clear
C)
vitamin B deficiency
done
clear
D)
vitamin A deficiency
done
clear
View Answer play_arrow
question_answer 172) In our body, the blood bank is
A)
heart
done
clear
B)
liver
done
clear
C)
spleen
done
clear
D)
red bone marrow
done
clear
View Answer play_arrow
question_answer 173) Main cause of beri-beri disease is
A)
vitamin \[{{B}_{12}}\] deficiency
done
clear
B)
vitamin \[{{B}_{1}}\] deficiency
done
clear
C)
vitamin \[{{B}_{2}}\] deficiency
done
clear
D)
vitamin \[{{B}_{6}}\] deficiency
done
clear
View Answer play_arrow
question_answer 174) Sperm head mainly contains the
A)
mitochondria
done
clear
B)
centrosome
done
clear
C)
golgi body
done
clear
D)
nucleus
done
clear
View Answer play_arrow
question_answer 175) In males, the essential hormones for secondary sexual characteristics is
A)
relaxin
done
clear
B)
estrogen
done
clear
C)
progesterone
done
clear
D)
testosterone
done
clear
View Answer play_arrow
question_answer 176) Graffian follicle of ovary secretes which hormone
A)
cortisone
done
clear
B)
relaxin
done
clear
C)
progesterone
done
clear
D)
estrone
done
clear
View Answer play_arrow
question_answer 177) Cretinism is due to less secretion of
A)
adrenal gland
done
clear
B)
parathyroid gland
done
clear
C)
pituitary gland
done
clear
D)
thyroid gland
done
clear
View Answer play_arrow
question_answer 178) The nerve related with diaphragm is
A)
glossopharyngeal
done
clear
B)
trigeminal
done
clear
C)
phrenic
done
clear
D)
vagus
done
clear
View Answer play_arrow
question_answer 179) The disease caused by deficiency of iodine is
A)
tetany
done
clear
B)
goitre
done
clear
C)
myxoedema
done
clear
D)
cretinism
done
clear
View Answer play_arrow
question_answer 180) Third ventricle is present in
A)
liver
done
clear
B)
kidney
done
clear
C)
brain
done
clear
D)
heart
done
clear
View Answer play_arrow
question_answer 181) What is the function of amnion?
A)
Protection from shocks
done
clear
B)
Nutrition
done
clear
C)
Excretion
done
clear
D)
Respiration
done
clear
View Answer play_arrow
question_answer 182) In nephrons there is complete absorption of
A)
water
done
clear
B)
glucose
done
clear
C)
salt
done
clear
D)
urea
done
clear
View Answer play_arrow
question_answer 183) Fallopian tube is the part of
A)
vas defcrens
done
clear
B)
oviduct
done
clear
C)
ureter
done
clear
D)
uterus
done
clear
View Answer play_arrow
question_answer 184) Lymph (nodes) glands form
A)
antibodies
done
clear
B)
antigens
done
clear
C)
lymphs
done
clear
D)
hormones
done
clear
View Answer play_arrow
question_answer 185) Movement in sperm is by
A)
tail
done
clear
B)
middle piece
done
clear
C)
acrosome
done
clear
D)
head
done
clear
View Answer play_arrow
question_answer 186) The vitamin which is essential for blood clotting is
A)
vitamin K
done
clear
B)
vitamin D
done
clear
C)
vitamin B
done
clear
D)
vitamin A
done
clear
View Answer play_arrow
question_answer 187) Ear drum is known as
A)
scala vcstibuli
done
clear
B)
scala tympani
done
clear
C)
tensor tympani
done
clear
D)
tympanic membrane
done
clear
View Answer play_arrow
question_answer 188) Universal donor is
A)
B blood group
done
clear
B)
A blood group
done
clear
C)
AB blood group
done
clear
D)
O blood group
done
clear
View Answer play_arrow
question_answer 189) AIDS causing factors are associated with
A)
Protozoa
done
clear
B)
Bacteria
done
clear
C)
DNA virus
done
clear
D)
RNA virus
done
clear
View Answer play_arrow
question_answer 190) Nails are formed by
A)
keratin
done
clear
B)
cartilage
done
clear
C)
chitin
done
clear
D)
bones
done
clear
View Answer play_arrow
question_answer 191) Which disease is caused by activation of oncogenes?
A)
Viral flu
done
clear
B)
T.B.
done
clear
C)
Cancer
done
clear
D)
Cholera
done
clear
View Answer play_arrow
question_answer 192) Striped muscles are
A)
anucleate
done
clear
B)
binucleale
done
clear
C)
uninucleate
done
clear
D)
syncytial
done
clear
View Answer play_arrow
question_answer 193) Haemozoin is a toxic substance formed in case of malaria. It is produced by
A)
cryptozoites
done
clear
B)
dead WBC
done
clear
C)
colour pigment of RBC
done
clear
D)
globin protein of RBC
done
clear
View Answer play_arrow
question_answer 194) Striped muscles are present in
A)
limb muscles
done
clear
B)
blood vessels
done
clear
C)
gall bladder
done
clear
D)
lungs
done
clear
View Answer play_arrow
question_answer 195) Whose secretion forms the pearl?
A)
Connective tissue of mantle
done
clear
B)
Ciliated epithelial cells of mantle
done
clear
C)
Columnar epithelial cell of mantle
done
clear
D)
Prismatic layer
done
clear
View Answer play_arrow
question_answer 196) Alcohol addiction is harmful because it causes
A)
cancer
done
clear
B)
rise in blood sugar level
done
clear
C)
deposition of extra fat in liver
done
clear
D)
protein deposition in liver
done
clear
View Answer play_arrow
question_answer 197) Which scientist gave the theory of continuity of germplasm?
A)
Darwin
done
clear
B)
Lamarck
done
clear
C)
Mendel
done
clear
D)
Weismann
done
clear
View Answer play_arrow
question_answer 198) Who wrote the book Genetics and the Origin of species
A)
Fischer
done
clear
B)
Julian Huxley
done
clear
C)
Dobzhansky
done
clear
D)
Devis
done
clear
View Answer play_arrow
question_answer 199) Proper burial of dead bodies, for the first time, started in which pre-historic mans period?
A)
Cromagnon man
done
clear
B)
Neanderthal man
done
clear
C)
Java man
done
clear
D)
Peking man
done
clear
View Answer play_arrow
question_answer 200) In honey bee, drones are produced by
A)
larva fed with royal jelly
done
clear
B)
low fed larvae
done
clear
C)
fertilized eggs
done
clear
D)
unfertilized eggs
done
clear
View Answer play_arrow