question_answer 1) The internal resistance of a cell is the resistance of:
A)
electrolyte used in cell
done
clear
B)
electrodes of the cell
done
clear
C)
material used in cell
done
clear
D)
vessel of the cell
done
clear
View Answer play_arrow
question_answer 2) A device for generating an alternating current of a desired frequency is known as:
A)
an oscillator
done
clear
B)
an amplifier
done
clear
C)
a rectifier
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 3) The angle of minimum deviation for a thin prism with respect to air and when dipped in water will be:\[\left( {{\,}_{a}}{{\mu }_{g}}=\frac{3}{2}{{,}_{a}}{{\mu }_{w}}=\frac{4}{3} \right)\]
A)
\[\frac{1}{4}\]
done
clear
B)
\[\frac{1}{8}\]
done
clear
C)
\[\frac{1}{3}\]
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 4) Escape velocity of a body when projected from the earths surface is 11.2 km/s. If it is projected at an angle of \[\text{5}{{\text{0}}^{\text{o}}}\] from the horizontal, the escape velocity is:
A)
11.8 km/s
done
clear
B)
16.5 km/s
done
clear
C)
11.2 km/s
done
clear
D)
14.5 km/s.
done
clear
View Answer play_arrow
question_answer 5) A diamagnetic substance is brought near a strong magnet, then it is:
A)
attracted by a magnet
done
clear
B)
repelled by a magnet
done
clear
C)
repelled by north pole and attracted by south pole
done
clear
D)
attracted by north pole and repelled by south pole
done
clear
View Answer play_arrow
question_answer 6) The ratio of intensities of two waves is 9:16. If they interfere, the ratio of maximum to minimum intensity will be:
A)
4 : 1
done
clear
B)
1 : 25
done
clear
C)
1 : 3
done
clear
D)
49 : 1
done
clear
View Answer play_arrow
question_answer 7) The sun emits a light with maximum wavelength 510 nm while another star emits a light with maximum wavelength of 350 nm. The ratio of surface temperature of sun and the star will be:
A)
0.69
done
clear
B)
0.46
done
clear
C)
1.45
done
clear
D)
2.1
done
clear
View Answer play_arrow
question_answer 8) If the equation of motion of standing wave is \[y=0.3\,\sin (314\,t-1.57x),\]then the velocity of standing wave is:
A)
400 unit
done
clear
B)
350 unit
done
clear
C)
209 unit
done
clear
D)
200 unit
done
clear
View Answer play_arrow
question_answer 9) For driving current of 2 A for 6 min in a circuit, 1000 J of work is to be done. The emf of the source in the circuit is:
A)
2.03 V
done
clear
B)
2.54 V
done
clear
C)
1:25 V
done
clear
D)
1.39 V
done
clear
View Answer play_arrow
question_answer 10) The moment of momentum for an electron in second orbit of hydrogen atom as per Bohrs model is:
A)
\[\frac{h}{\pi }\]
done
clear
B)
\[2\pi h\]
done
clear
C)
\[\frac{2h}{\pi }\]
done
clear
D)
\[\frac{\pi }{h}\]
done
clear
View Answer play_arrow
question_answer 11) The latent heat of vaporisation of water is \[\text{2250}\,\text{J/kg}\text{.}\]If the work done in the process of vaporisation of 1 kg is 168 J, then increase in internal energy will be:
A)
1904 J
done
clear
B)
1984 J
done
clear
C)
3202 J
done
clear
D)
2082 J
done
clear
View Answer play_arrow
question_answer 12) Huygens wave theory ,of light could not explain:
A)
photoelectric effect
done
clear
B)
interference
done
clear
C)
diffraction
done
clear
D)
polarization
done
clear
View Answer play_arrow
question_answer 13) In a triode valve, when the plate potential is increased from 200 V to 220 V and grid potential is decreased from \[-0.5\text{ V}\]to \[-\text{ 1}\text{.3 V,}\] there is 1 no change in plate current. The amplification factor of the triode is:
A)
25
done
clear
B)
14
done
clear
C)
11
done
clear
D)
73
done
clear
View Answer play_arrow
question_answer 14) A bar magnet of magnetic moment \[\text{220 A}{{\text{m}}^{\text{2}}}\]is suspended in a magnetic field of intensity 0.25 N/Am. The couple required to deflect it through \[\text{3}{{\text{0}}^{\text{o}}}\]is:
A)
27.5 Nm
done
clear
B)
20.25 Nm
done
clear
C)
47.63 Nm
done
clear
D)
12 Nm
done
clear
View Answer play_arrow
question_answer 15) A plano-convex lens has refractive index 1.5 and radius of curvature 50 cm. What is focal length of lens?
A)
100 cm
done
clear
B)
200 cm
done
clear
C)
178 cm
done
clear
D)
150 cm
done
clear
View Answer play_arrow
question_answer 16) A source and an observer move away from each other, with a velocity of 20 m/s. If the apparent frequency heard by the observer is 1840 Hz, the actual frequency of the source is: (Velocity of sound in air = 340 m/s):
A)
2486 Hz
done
clear
B)
2070 Hz
done
clear
C)
2134 Hz
done
clear
D)
1872 Hz
done
clear
View Answer play_arrow
question_answer 17) The speed of wave in a medium is 650 m/s. If 3500 waves are passing through a point in the medium in 1.67 min, then its wavelength will be:
A)
16.25m
done
clear
B)
14.29m
done
clear
C)
18.57m
done
clear
D)
20.50m
done
clear
View Answer play_arrow
question_answer 18) The body is projected at such angle that the horizontal range is three times the greatest height. The angle of projection is:
A)
\[{{43}^{o}}8\]
done
clear
B)
\[{{25}^{o}}8\]
done
clear
C)
\[{{33}^{o}}7\]
done
clear
D)
\[{{53}^{o}}1\]
done
clear
View Answer play_arrow
question_answer 19) On the horizontal surface of a truck, a block of mass 1 kg is placed \[(\mu =0.6)\] and truck is moving with acceleration \[\text{5}\,\text{m/}{{\text{s}}^{\text{2}}}\text{.}\] The frictional force acting on the block will be:\[(g=10\,m/{{s}^{2}})\]
A)
6 N
done
clear
B)
5.88 N
done
clear
C)
7 N
done
clear
D)
9 N
done
clear
View Answer play_arrow
question_answer 20) Calculate the work done when a force \[\vec{F}=2\hat{i}+3\hat{j}-5\hat{k}\]units acts on a body producing a displacement\[\vec{s}=2\hat{i}+4\hat{j}+3\hat{k}\] units:
A)
1 unit
done
clear
B)
20 unit
done
clear
C)
5 unit
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 21) The efficiency of Car not engine is 50% and temperature of sink is 500 K. If the temperature of source is kept constant and its efficiency is to be raised to 60%, then the required temperature of the sink will be:
A)
600 K
done
clear
B)
400 K
done
clear
C)
500 K
done
clear
D)
100 K
done
clear
View Answer play_arrow
question_answer 22) What happens to the internal energy of a gas during isothermal expansion?
A)
Internal energy will decrease
done
clear
B)
Internal energy: will increase
done
clear
C)
Internal energy will become zero
done
clear
D)
Internal energy will remain same
done
clear
View Answer play_arrow
question_answer 23) 100 g ice is mixed with 100 g of water at \[100{{\,}^{o}}C.\] What will be the final temperature of the mixture?
A)
\[~10{{\,}^{o}}C\]
done
clear
B)
\[~27{{\,}^{o}}C\]
done
clear
C)
\[~14{{\,}^{o}}C\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 24) Black hole consists of:
A)
ozone layer
done
clear
B)
super dense planetary material
done
clear
C)
upper surface of atmosphere
done
clear
D)
none of the above
done
clear
View Answer play_arrow
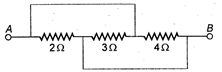
question_answer 25)
Three resistors \[2\Omega ,\,3\Omega \] and \[4\Omega \] are connected as shown in the given diagram. The equivalent resistance will be:
A)
\[\frac{12}{13}\]
done
clear
B)
\[\frac{11}{10}\]
done
clear
C)
2
done
clear
D)
\[\frac{10}{11}\]
done
clear
View Answer play_arrow
question_answer 26) If an electron jumps from first orbit to third orbit of hydrogen atom it will:
A)
not loose energy
done
clear
B)
release energy
done
clear
C)
absorb energy
done
clear
D)
not gain energy
done
clear
View Answer play_arrow
question_answer 27) A body of mass 10 kg moving with velocity 10 m/s collides with a stationary body of mass 5 kg. After collision both bodies stick to each other, velocity of the joint body after collision is:
A)
\[\frac{3}{10}\,m/s\]
done
clear
B)
\[20\,m/s\]
done
clear
C)
\[\frac{20}{3}\,m/s\]
done
clear
D)
\[15\,m/s\]
done
clear
View Answer play_arrow
question_answer 28) If a body starts from rest and travels 110 cm in the 9th second, then acceleration of the body is:
A)
\[0.13\text{ }m/{{s}^{2}}\]
done
clear
B)
\[~0.16\text{ }m/{{s}^{2}}\]
done
clear
C)
\[~0.18\text{ m/}{{\text{s}}^{2}}\]
done
clear
D)
\[~0.34\text{ }m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 29) If the momentum of a particle is doubled, then its de-Broglie wavelength will:
A)
become four times
done
clear
B)
become half
done
clear
C)
become two times
done
clear
D)
remain unchanged
done
clear
View Answer play_arrow
question_answer 30) Dimensions of torque are:
A)
\[[{{M}^{2}}{{L}^{2}}{{T}^{-2}}]\]
done
clear
B)
\[[M{{L}^{2}}{{T}^{-2}}]\]
done
clear
C)
\[[M{{L}^{2}}{{T}^{-1}}]\]
done
clear
D)
\[[M{{L}^{0}}{{T}^{-2}}]\]
done
clear
View Answer play_arrow
question_answer 31) Two vectors have magnitudes 3 and 5. If angle between them is \[\text{6}{{\text{0}}^{\text{o}}}\text{,}\] then the dot product of two vectors will be:
A)
7.5
done
clear
B)
6.5
done
clear
C)
8.4
done
clear
D)
7.9
done
clear
View Answer play_arrow
question_answer 32) The kinetic energy of 1 g molecule of a gas, at normal temperature and pressure is:\[\text{(R}\,\text{=}\,\text{8}\text{.321}\,\text{J/mol-K)}\]
A)
\[1.2\times {{10}^{2}}J\]
done
clear
B)
\[3.4\times {{10}^{3}}J\]
done
clear
C)
\[1.66\times {{10}^{4}}J\]
done
clear
D)
\[2.97\times {{10}^{4}}J\]
done
clear
View Answer play_arrow
question_answer 33) If the coefficient of cubical expansion is \[x\]times of the coefficient of superficial expansion, then value of \[x\] is:
A)
2.7
done
clear
B)
2
done
clear
C)
1.5
done
clear
D)
9.5
done
clear
View Answer play_arrow
question_answer 34) X-ray will not show the phenomenon of:
A)
interference
done
clear
B)
deflection by electric field
done
clear
C)
diffraction
done
clear
D)
polarization
done
clear
View Answer play_arrow
question_answer 35) Bernoullis theorem is based on:
A)
conservation of mass, energy and momentum
done
clear
B)
conservation of mass
done
clear
C)
conservation of momentum
done
clear
D)
conservation of energy
done
clear
View Answer play_arrow
question_answer 36) In bringing an electron towards another electron, the electrostatic potential energy of the system:
A)
decreases
done
clear
B)
increases
done
clear
C)
remains same
done
clear
D)
becomes zero
done
clear
View Answer play_arrow
question_answer 37) The half-life of radium is 1600 yr. What is the mean life and disintegration constant of radium?
A)
\[2309\,yr,\,\frac{1}{2309}/yr\,\]
done
clear
B)
\[3309\,yr,\,\frac{1}{3309}/yr\,\]
done
clear
C)
\[1309\,yr,\,\frac{1}{1309}/yr\,\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 38) Energy obtained when 1 mg mass is completely converted in to energy will be:
A)
\[9\times {{10}^{13}}\,J\]
done
clear
B)
\[3\times {{10}^{8}}\,J\]
done
clear
C)
\[3\times {{10}^{15}}\,J\]
done
clear
D)
\[9\times {{10}^{15}}\,J\]
done
clear
View Answer play_arrow
question_answer 39) A ray of light is incident on a surface of a plate of glass of refractive index 1.5 at polarising angle. The angle of refraction of the ray will be:
A)
\[{{53.7}^{o}}\]
done
clear
B)
\[~{{43.7}^{o}}\]
done
clear
C)
\[{{33.7}^{o}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 40) A closed organ pipe and an open organ pipe are tuned to the same fundamental frequency. The ratio of their lengths is:
A)
1 : 2
done
clear
B)
4 : 1
done
clear
C)
1 : 4
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 41) A particle is moving along a circular path of radius 5m with uniform speed 5 m/s. What will be the average acceleration -when the particle completes half revolution?
A)
\[\frac{10}{\pi }m/{{s}^{2}}\]
done
clear
B)
\[10\,m/{{s}^{2}}\]
done
clear
C)
\[10\pi \,m/{{s}^{2}}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
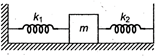
question_answer 42)
A body of mass m is attached between two springs of force constants \[{{k}_{1}}\]and \[{{k}_{2}}\]as shown in figure. The other ends of the springs are fixed to firm supports. The frequency of the oscillation is:
A)
\[n=\frac{1}{2\pi }\sqrt{\frac{{{k}_{1}}-{{k}_{2}}}{m}}\]
done
clear
B)
\[n=\frac{1}{2\pi }\sqrt{\frac{{{k}_{1}}{{k}_{2}}}{m}}\]
done
clear
C)
\[n=\frac{1}{2\pi }\sqrt{\frac{{{k}_{1}}+{{k}_{2}}}{m}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 43) In a p-type semiconductor, germanium is doped with:
A)
boron
done
clear
B)
gallium
done
clear
C)
aluminium
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 44) In a full wave rectifier circuit operating from 50 Hz mains frequency, what is the fundamental frequency in the ripple?
A)
\[50\,Hz\]
done
clear
B)
\[100\,Hz\]
done
clear
C)
\[25\,Hz\]
done
clear
D)
\[70\,Hz\]
done
clear
View Answer play_arrow
question_answer 45) An ideal gas at \[27{{\,}^{o}}C\]is compressed adiabatically to \[\frac{8}{27}\] of its original volume. The rise in temperature will be \[\left( \gamma =\frac{5}{3} \right):\]
A)
\[~480{{\,}^{o}}C\]
done
clear
B)
\[~275{{\,}^{o}}C\]
done
clear
C)
\[~450{{\,}^{o}}C\]
done
clear
D)
\[~375{{\,}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 46) When you make ice cubes, entropy of water:
A)
does not change
done
clear
B)
increases
done
clear
C)
decreases
done
clear
D)
may either increase or decrease depending on the process used
done
clear
View Answer play_arrow
question_answer 47) The focal length of the objective lens and eye-piece of an astronomical telescope are 2m and 0.05 m. Find the length of the telescope.
A)
2.05 m
done
clear
B)
1.16 m
done
clear
C)
1.05 m
done
clear
D)
2.9 m
done
clear
View Answer play_arrow
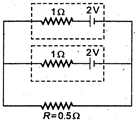
question_answer 48)
Two identical batteries, each of emf 2 V and internal resistance \[1\,\Omega \]pass a current through external resistance \[R=0.5\,\Omega .\]The maximum power that can be developed across R using these batteries is:
A)
3.2W
done
clear
B)
8.2W
done
clear
C)
4 W
done
clear
D)
2 W
done
clear
View Answer play_arrow
question_answer 49) At what height from the earths surface, the acceleration due to gravity will be half the value of g at surface? (R= 6400 km)
A)
6400 km
done
clear
B)
8200 km
done
clear
C)
4800 km
done
clear
D)
1600 km
done
clear
View Answer play_arrow
question_answer 50) In a semiconducting material the mobilities of electrons and holes are \[{{\mu }_{e}}\]and \[{{\mu }_{h}}\] respectively which of the following is true?
A)
\[{{\mu }_{e}}>{{\mu }_{h}}\]
done
clear
B)
\[{{\mu }_{e}}<{{\mu }_{h}}\]
done
clear
C)
\[{{\mu }_{e}}={{\mu }_{h}}\]
done
clear
D)
\[{{\mu }_{e}}<0,\,{{\mu }_{h}}>0\]
done
clear
View Answer play_arrow
question_answer 51) Tyndall effect is shown by:
A)
solution
done
clear
B)
precipitate
done
clear
C)
sol
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 52) In reaction, \[MnO_{4}^{-}+{{H}^{+}}+{{C}_{2}}O_{4}^{2-}\xrightarrow{{}}M{{n}^{2+}}\] \[+\,{{H}_{2}}O+C{{O}_{2}}\] What is happening?
A)
Reduction of Mn
done
clear
B)
Reduction of \[{{\text{C}}_{\text{2}}}\text{O}_{4}^{2-}\]
done
clear
C)
Oxidation of Mn
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 53) In which of the following ionic bond is present?
A)
\[B{{F}_{3}}\]
done
clear
B)
\[CC{{l}_{4}}\]
done
clear
C)
\[HCl\]
done
clear
D)
\[BC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 54) Deficiency of vitamin E causes:
A)
scurvy
done
clear
B)
beri-beri
done
clear
C)
sterility
done
clear
D)
xerophthalmia
done
clear
View Answer play_arrow
question_answer 55) The reaction of Lucas reagent is fast with:
A)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
B)
\[{{(C{{H}_{3}})}_{3}}COH\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}CHOH\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
View Answer play_arrow
question_answer 56) IUPAC name of the compound is: \[H-\overset{\text{O}}{\mathop{\overset{||}{\mathop{C}}\,}}\,-C{{H}_{2}}-C{{H}_{2}}-COOH\]
A)
3-oxo-butanoic acid
done
clear
B)
4-oxo-propanoic acid
done
clear
C)
3-formyl-propanoic acid
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 57) Tollens reagent is:
A)
alkaline \[\text{CuS}{{\text{O}}_{\text{4}}}\]solution
done
clear
B)
ammoniacal cuproxide solution
done
clear
C)
aqueous solution of sodium cupritartarate
done
clear
D)
ammoniacal \[\text{AgN}{{\text{O}}_{\text{3}}}\]solution
done
clear
View Answer play_arrow
question_answer 58) Epsom salt is:
A)
\[MgS{{O}_{4}}.2{{H}_{2}}O\]
done
clear
B)
\[BaS{{O}_{4}}.2{{H}_{2}}O\]
done
clear
C)
\[MgS{{O}_{4}}.7{{H}_{2}}O\]
done
clear
D)
\[CaS{{O}_{4}}.2{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 59) Which of the following carbonate decompose most easily on heating?
A)
\[R{{b}_{2}}C{{O}_{3}}\]
done
clear
B)
\[{{K}_{2}}C{{O}_{3}}\]
done
clear
C)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
D)
\[MgC{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 60) In an isothermal expansion of an ideal gas:
A)
\[W=0\]
done
clear
B)
\[\Delta E=0\]
done
clear
C)
\[q=0\]
done
clear
D)
\[\Delta V=0\]
done
clear
View Answer play_arrow
question_answer 61) The degree of hydrolysis of \[\text{0}\text{.01 M N}{{\text{H}}_{\text{4}}}\text{C1}\]is:\[({{K}_{h}}=2.5\times {{10}^{-9}})\]
A)
\[5\times {{10}^{-5}}\]
done
clear
B)
\[5\times {{10}^{-4}}\]
done
clear
C)
\[5\times {{10}^{-3}}\]
done
clear
D)
\[5\times {{10}^{-7}}\]
done
clear
View Answer play_arrow
question_answer 62) The number of gram equivalent of \[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]in 1000 mL 3M solution, is:
A)
3
done
clear
B)
6
done
clear
C)
4
done
clear
D)
1.5
done
clear
View Answer play_arrow
question_answer 63) The radioactive isotope used in cancer therapy, is
A)
\[{{1}^{128}}\]
done
clear
B)
\[C{{o}^{60}}\]
done
clear
C)
\[C{{o}^{59}}\]
done
clear
D)
\[{{P}^{32}}\]
done
clear
View Answer play_arrow
question_answer 64) The expression of angular momentum of an electron in a Bohrs orbit is:
A)
\[\frac{n}{3}\frac{h}{\pi }\]
done
clear
B)
\[\frac{n}{2}\frac{h}{\pi }\]
done
clear
C)
\[\frac{h}{4\pi }\]
done
clear
D)
\[\sqrt{l(l+1)}.\frac{h}{2\pi }\]
done
clear
View Answer play_arrow
question_answer 65) Which of the following is called Hofmanns bromamide reaction?
A)
\[C{{H}_{3}}CN+2{{H}_{2}}O\xrightarrow{{}}C{{H}_{3}}COO+N{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}CN+4H\xrightarrow{Na/EtOH}C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}CON{{H}_{2}}\xrightarrow{B{{r}_{2}}/NaOH}C{{H}_{3}}N{{H}_{2}}\]
done
clear
D)
\[C{{H}_{2}}COCl+C{{H}_{3}}OH\xrightarrow{{}}\]\[C{{H}_{3}}COOC{{H}_{3}}+HCl\]
done
clear
View Answer play_arrow
question_answer 66) Which one among the following contains a phenolic-OH group?
A)
Oxalic acid
done
clear
B)
Formic acid
done
clear
C)
Picric acid
done
clear
D)
Citric acid
done
clear
View Answer play_arrow
question_answer 67) In the reaction, product X is: \[C{{H}_{3}}-C\equiv CH+{{H}_{2}}O\xrightarrow{{{H}^{+}}/H{{g}^{2+}}}X\]
A)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}COOH\]
done
clear
C)
\[C{{H}_{3}}-C(OH)=CHOH\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 68) Which is an amphoteric oxide?
A)
\[MgO\]
done
clear
B)
\[{{K}_{2}}O\]
done
clear
C)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
D)
\[CuO\]
done
clear
View Answer play_arrow
question_answer 69) The oxidation number of S in \[{{\text{H}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{8}}}\]is:
A)
\[+\,6\]
done
clear
B)
\[-\,6\]
done
clear
C)
\[+\,\,4\]
done
clear
D)
\[+\,\,8\]
done
clear
View Answer play_arrow
question_answer 70) Bleaching powder is obtained by treating chlorine with:
A)
\[CaC{{O}_{3}}\]
done
clear
B)
\[Ca{{(OH)}_{2}}\]
done
clear
C)
\[CaO\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 71) For reaction, \[PC{{l}_{3}}(g)+C{{l}_{2}}(g)PC{{l}_{5}}(g)\] the value of \[{{K}_{c}}\]at \[\text{250}{{\,}^{\text{o}}}\text{C}\]is 26. At the same temperature, the value of \[{{K}_{p}}\]is:
A)
0.46
done
clear
B)
0.61
done
clear
C)
0.95
done
clear
D)
0.73
done
clear
View Answer play_arrow
question_answer 72) The volume of a gas is reduced to 1.0 L at \[\text{25}{{\,}^{\text{o}}}\text{C}\]and 1 atm. pressure. Its pressure at \[\text{35}{{\,}^{\text{o}}}\text{C}\]would be:
A)
0.96 atm
done
clear
B)
1.03 atm
done
clear
C)
2.04 atm
done
clear
D)
3.08 atm
done
clear
View Answer play_arrow
question_answer 73) The strongest acid among the following is:
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{2}}ClCOOH\]
done
clear
C)
\[C{{H}_{2}}FCOOH\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}COOH\]
done
clear
View Answer play_arrow
question_answer 74) The compressibility factor for an ideal gas is:
A)
\[<1\]
done
clear
B)
\[=1\]
done
clear
C)
\[>1\]
done
clear
D)
always 2
done
clear
View Answer play_arrow
question_answer 75) The standard reduction potential of \[\text{L}{{\text{i}}^{\text{+}}}\text{,}\,\text{B}{{\text{a}}^{\text{2+}}}\text{,}\,\text{N}{{\text{a}}^{\text{+}}}\]and \[\text{M}{{\text{g}}^{2+}}\]are \[-3.05,-2.73,\]\[-2.71\]and \[-2.37\] volts respectively. Which one is the strongest reducing agent?
A)
Li
done
clear
B)
Na
done
clear
C)
Mg
done
clear
D)
Ba
done
clear
View Answer play_arrow
question_answer 76) The unit of rate constant for 1st order reaction is:
A)
\[mol\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
B)
\[mo{{l}^{-1}}\,L{{s}^{-1}}\]
done
clear
C)
\[{{s}^{-1}}\]
done
clear
D)
\[mo{{l}^{2}}{{L}^{-2}}{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 77) In which of the following, the dipole moment is zero?
A)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[S{{O}_{2}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 78) The bond order of \[\text{O}_{\text{2}}^{\text{+}}\] is:
A)
2
done
clear
B)
2.5
done
clear
C)
1.5
done
clear
D)
0.5
done
clear
View Answer play_arrow
question_answer 79) Addition of \[\text{H}\text{gC}{{\text{l}}_{\text{2}}}\]to \[\text{SnC}{{\text{l}}_{\text{2}}}\]gives a black colour due to:
A)
oxidation of Sn
done
clear
B)
reduction of \[\text{HgC}{{\text{l}}_{\text{2}}}\]
done
clear
C)
formation of amalgum
done
clear
D)
oxidation of Hg
done
clear
View Answer play_arrow
question_answer 80) Which one of the following is a trihydric alcohol containing only secondary hydroxyl group?
A)
\[C{{H}_{3}}-\underset{OH}{\overset{C{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{OH}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-C{{H}_{2}}OH\]
done
clear
B)
\[\underset{OH}{\mathop{\underset{|}{\mathop{C{{H}_{2}}}}\,}}\,-\underset{OH}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-\underset{OH}{\mathop{\underset{|}{\mathop{C{{H}_{2}}}}\,}}\,\]
done
clear
C)
\[C{{H}_{3}}-\underset{OH}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-\underset{OH}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-\underset{OH}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-C{{H}_{3}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 81) How many sigma and n-bonds are there in the following compound? \[C{{H}_{2}}=CH-C\equiv C-\overset{C{{H}_{3}}}{\mathop{\overset{|}{\mathop{C}}\,}}\,=C{{H}_{2}}\]
A)
\[11\sigma ,4\pi \]
done
clear
B)
\[12\sigma ,3\pi \]
done
clear
C)
\[14\sigma ,4\pi \]
done
clear
D)
\[15\sigma ,4\pi \]
done
clear
View Answer play_arrow
question_answer 82) In the nitration of benzene, the reactive species is:
A)
\[N{{O}_{2}}\]
done
clear
B)
\[NO_{2}^{+}\]
done
clear
C)
\[NO_{2}^{-}\]
done
clear
D)
\[N{{O}^{-}}\]
done
clear
View Answer play_arrow
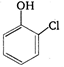
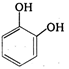
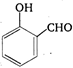
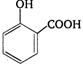
question_answer 83) Reaction of phenol with \[\text{CC}{{\text{l}}_{\text{4}}}\]and\[\text{NaOH,}\] followed by hydrolysis is likely to give:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 84) In the reaction,\[{{\,}_{\text{92}}}{{\text{U}}^{\text{238}}}\xrightarrow{{}}{{\,}_{\text{82}}}\text{P}{{\text{b}}^{\text{206}}}\text{,}\] the number of\[\alpha \]and \[\beta -\]particles emitted are:
A)
\[7\alpha ,5\beta \]
done
clear
B)
\[6\alpha ,4\beta \]
done
clear
C)
\[4\alpha ,3\beta \]
done
clear
D)
\[8\alpha ,6\beta \]
done
clear
View Answer play_arrow
question_answer 85) Which is the correct Gibbs Helmholtz equation?
A)
\[\Delta H=\Delta G-T.\Delta S\]
done
clear
B)
\[\Delta S=\frac{1}{T}[\Delta G-\Delta H]\]
done
clear
C)
\[\Delta S=\frac{1}{T}[\Delta H-\Delta G]\]
done
clear
D)
\[-\Delta G=\Delta H-T.\Delta S\]
done
clear
View Answer play_arrow
question_answer 86) Vinegar is:
A)
8-10% acetic acid,
done
clear
B)
6-10% ethyl alcohol
done
clear
C)
glacial acetic acid
done
clear
D)
10% formic acid
done
clear
View Answer play_arrow
question_answer 87) Decreasing order of - electron affinity of halogens is:
A)
\[Cl>Br>F>I\]
done
clear
B)
\[Cl>F>Br>I\]
done
clear
C)
\[Br>Cl>F>I\]
done
clear
D)
\[F>Cl>Br>I\]
done
clear
View Answer play_arrow
question_answer 88) Which of the following is an acidic salt?
A)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
B)
\[Pb(OH)Cl\]
done
clear
C)
\[BaC{{l}_{2}}\]
done
clear
D)
\[N{{a}_{2}}HP{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 89) Which one is the Lewis acid?
A)
\[{{H}_{2}}O\]
done
clear
B)
\[AlC{{l}_{3}}\]
done
clear
C)
\[RN{{H}_{2}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 90) 720 g water contain the number of moles:
A)
2
done
clear
B)
190
done
clear
C)
40
done
clear
D)
55
done
clear
View Answer play_arrow
question_answer 91) \[2HCHO\xrightarrow{50%\,NaOH}C{{H}_{3}}OH+HCOONa\] This reaction is called:
A)
aldol condensation
done
clear
B)
Tischenko reaction
done
clear
C)
Cannizaro reaction
done
clear
D)
Reimer Tiemann reaction
done
clear
View Answer play_arrow
question_answer 92) The geometry of the molecule having s and \[p-\]characters equal to 25% and 75% respectively, is:
A)
trigonal
done
clear
B)
linear
done
clear
C)
tetrahedral
done
clear
D)
octahedral
done
clear
View Answer play_arrow
question_answer 93) Impossible configuration is:
A)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{2}},3{{s}^{2}}\]
done
clear
B)
\[1{{s}^{2}},2{{s}^{2}}\]
done
clear
C)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 94) The solubility product of \[\text{PbB}{{\text{r}}_{\text{2}}}\]is \[\text{10}\text{.8}\times {{10}^{-5}}.\] It is 70% dissociated in saturated solution. The solubility of salt is:
A)
\[4.18\times {{10}^{-2}}\]
done
clear
B)
\[6.76\times {{10}^{-3}}\]
done
clear
C)
\[3.4\times {{10}^{-4}}\]
done
clear
D)
\[5.44\times {{10}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 95) The latent heat of vaporisation of water is \[540\,\text{cal}\,{{\text{g}}^{-1}}\]at \[\text{100}{{\,}^{\text{o}}}\text{C}\text{.}\] What will be the entropy increase when one mole of water is evaporated at \[\text{100}{{\,}^{\text{o}}}\text{C?}\]
A)
\[28\,cal\,{{K}^{-1}}\,mo{{l}^{-1}}\]
done
clear
B)
\[26\,cal\,{{K}^{-1}}\,mo{{l}^{-1}}\]
done
clear
C)
\[540\,cal\,{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
D)
\[1.82\,cal\,{{K}^{-1}}mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 96) The brown ring in the test of nitrate is formed due to:
A)
\[{{[Fe{{({{H}_{2}}O)}_{5}}N{{O}_{2}}]}^{2+}}\]
done
clear
B)
\[Fe{{(N{{O}_{3}})}_{3}}\]
done
clear
C)
\[{{[Fe{{({{H}_{2}}O)}_{5}}NO]}^{2+}}\]
done
clear
D)
\[{{[Fe({{H}_{2}}O){{(NO)}_{5}}]}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 97) The corrosive sublimate is:
A)
Hg
done
clear
B)
\[H{{g}_{2}}O\]
done
clear
C)
\[H{{g}_{2}}C{{l}_{2}}\]
done
clear
D)
\[HgC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 98) The half-life of radium is 1580 yr. Its average life is:
A)
\[2.275\times {{10}^{3}}yr\]
done
clear
B)
\[3.735\times {{10}^{2}}yr\]
done
clear
C)
\[1.62\times {{10}^{3}}yr\]
done
clear
D)
\[7.28\times {{10}^{2}}yr\]
done
clear
View Answer play_arrow
question_answer 99) Cobalt is present in:
A)
vitamin\[{{B}_{12}}\]
done
clear
B)
vitamin\[{{B}_{6}}\]
done
clear
C)
vitamin \[{{B}_{2}}\]
done
clear
D)
vitamin\[{{B}_{1}}\]
done
clear
View Answer play_arrow
question_answer 100) Adipic acid and hexamethylene diamine, on polymerisation gives:
A)
nylon-6
done
clear
B)
Dacron
done
clear
C)
nylon-66
done
clear
D)
Bakelite
done
clear
View Answer play_arrow
question_answer 101) Reserpine is obtained from:
A)
Asafoetida
done
clear
B)
Rauwolfia serpentine
done
clear
C)
Curcuma longa
done
clear
D)
Papaver somniferum
done
clear
View Answer play_arrow
question_answer 102) Seed coat is derived from:
A)
pericarp
done
clear
B)
epicarp
done
clear
C)
integuments of ovule
done
clear
D)
nucellus
done
clear
View Answer play_arrow
question_answer 103) A characteristic feature of ovary of Brassica compesrris is:
A)
presence of replum
done
clear
B)
axile placentation
done
clear
C)
epigynous
done
clear
D)
multilocular nature
done
clear
View Answer play_arrow
question_answer 104) One of the characteristic of Hydra is:
A)
predation
done
clear
B)
metamerism
done
clear
C)
hibernation
done
clear
D)
mimicry
done
clear
View Answer play_arrow
question_answer 105) Which of the following pair lack the unit membrane?
A)
Nucleus and ER
done
clear
B)
Mitochondria and chloroplast
done
clear
C)
Ribosomes and nucleolus
done
clear
D)
Golgi body and lysosome
done
clear
View Answer play_arrow
question_answer 106) The number of DNA in chromosome at \[{{\text{G}}_{2}}\] stage:
A)
one
done
clear
B)
two
done
clear
C)
four
done
clear
D)
eight
done
clear
View Answer play_arrow
question_answer 107) Which of the following is a disaccharide?
A)
Glucose
done
clear
B)
Fructose
done
clear
C)
Sucrose
done
clear
D)
Galactose
done
clear
View Answer play_arrow
question_answer 108) Hydrolysis of lipid yields:
A)
fatty acids and maltose
done
clear
B)
fatty acids and glycerol
done
clear
C)
mannose and glycerol
done
clear
D)
maltose and fatty acid
done
clear
View Answer play_arrow
question_answer 109) Ctenophora shows affinities with:
A)
Cnidaria
done
clear
B)
Aschelminthes
done
clear
C)
Cephalopoda
done
clear
D)
Turbelaria
done
clear
View Answer play_arrow
question_answer 110) Exception of MendeFs law is:
A)
independent assortment
done
clear
B)
linkage
done
clear
C)
purity of gametes
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 111) The position of protoxylem in leaf is:
A)
adaxial
done
clear
B)
abaxial
done
clear
C)
surrounded by metaxylem
done
clear
D)
lateral
done
clear
View Answer play_arrow
question_answer 112) In Pheretima, locomotion occurs with the help of:
A)
circular muscles
done
clear
B)
longitudinal muscles and setae
done
clear
C)
circular, longitudinal muscles and setae
done
clear
D)
parapodia
done
clear
View Answer play_arrow
question_answer 113) In Amoeba which controls the cytoplasmic osmality?
A)
nucleus
done
clear
B)
ectoplasm
done
clear
C)
biurets
done
clear
D)
contractile vacuole
done
clear
View Answer play_arrow
question_answer 114) Branched, aseptate, coenocytic mycelium present in:
A)
Aspergillus
done
clear
B)
Albugo
done
clear
C)
Penicillium
done
clear
D)
Erysiphe
done
clear
View Answer play_arrow
question_answer 115) The economically important plant of Malvaceae:
A)
Gossypium hirsutum
done
clear
B)
Hibiscus cannabis
done
clear
C)
Abelmoschus esculentus
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 116) The cells without nuclei are present in:
A)
vascular cambium
done
clear
B)
root hair
done
clear
C)
companion cell
done
clear
D)
members of sieve tube
done
clear
View Answer play_arrow
question_answer 117) Which of the following is not correctly matched?
A)
Rhinencephalon- Olfactory
done
clear
B)
Hypothalamus- Pituitary
done
clear
C)
Cerebellum- Balance
done
clear
D)
Medulla oblongata - Temperature regulation
done
clear
View Answer play_arrow
question_answer 118) The scientist who was awarded Nobel Prize in 1959 for in vitro synthesis of polyribonucleotide?
A)
Mendel
done
clear
B)
Calvin
done
clear
C)
Khorana
done
clear
D)
Ochoa
done
clear
View Answer play_arrow
question_answer 119) Which of the following have sunken stomata?
A)
Nerium
done
clear
B)
Mangifera
done
clear
C)
Hydrilla
done
clear
D)
Zeamays
done
clear
View Answer play_arrow
question_answer 120) When a plasmolysed cell is placed in a hypotonic solution then water will move inside the cell. Which force causes this?
A)
DPD
done
clear
B)
O.P
done
clear
C)
WP
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 121) Group of two or more than two plants species is called as:
A)
plant community
done
clear
B)
animal ecosystem
done
clear
C)
plant ecosystem
done
clear
D)
ecological niche
done
clear
View Answer play_arrow
question_answer 122) Which type of kidneys are found in amphibians?
A)
Holonephric
done
clear
B)
Mesonephric
done
clear
C)
Pronephric
done
clear
D)
Metanephric
done
clear
View Answer play_arrow
question_answer 123) Which part of cell have enzymes for glycolysis?
A)
Cytoplasm
done
clear
B)
Mitochondria
done
clear
C)
Golgi complex
done
clear
D)
Nucleus
done
clear
View Answer play_arrow
question_answer 124) Botanical name of species which cause white rust of crucifers?
A)
Peronospora parasitica
done
clear
B)
Puccinia graminis
done
clear
C)
Pythium debaryanum
done
clear
D)
Albugo Candida
done
clear
View Answer play_arrow
question_answer 125) Which of the following nerve supplies organ of Cord?
A)
Auditory
done
clear
B)
Olfactory
done
clear
C)
Trochlear
done
clear
D)
Vagus
done
clear
View Answer play_arrow
question_answer 126) A pond is a:
A)
biome
done
clear
B)
natural ecosystem
done
clear
C)
artificial ecosystem
done
clear
D)
community of plants and animals
done
clear
View Answer play_arrow
question_answer 127) Which one of the following body functions is not performed by kidneys?
A)
Excretion
done
clear
B)
Osmoregulation
done
clear
C)
Regulation of blood volume
done
clear
D)
Destruction of dead blood corpuscles
done
clear
View Answer play_arrow
question_answer 128) The study of fossils is called:
A)
Palynology
done
clear
B)
Palaeontology
done
clear
C)
Fossil systematic
done
clear
D)
Pharmacognosy
done
clear
View Answer play_arrow
question_answer 129) The characteristic of blue-green algae is:
A)
DNA without histone
done
clear
B)
nuclear membrane absent
done
clear
C)
70S ribosome
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 130) Classification of sponges is primarily based on the:
A)
body organisation
done
clear
B)
body plan
done
clear
C)
skeleton
done
clear
D)
canal system
done
clear
View Answer play_arrow
question_answer 131) In mammals corpus luteum is found in which organ?
A)
Brain
done
clear
B)
Ovary
done
clear
C)
Liver
done
clear
D)
Eyes
done
clear
View Answer play_arrow
question_answer 132) Which character is found only in mammals?
A)
Neck
done
clear
B)
Diaphragm
done
clear
C)
Optic lobes of brain
done
clear
D)
Tail
done
clear
View Answer play_arrow
question_answer 133) Which of the following is not correct for gastrulation?
A)
Archenteron is formed
done
clear
B)
All germinal layers are formed
done
clear
C)
Morphogenetic movements
done
clear
D)
Some blastomeres and blastocoel degenerate
done
clear
View Answer play_arrow
question_answer 134) The chemical formula of starch is:
A)
\[{{({{C}_{6}}{{H}_{10}}{{O}_{5}})}_{n}}\]
done
clear
B)
\[{{({{C}_{6}}{{H}_{12}}{{O}_{6}})}_{n}}\]
done
clear
C)
\[{{C}_{12}}{{H}_{22}}{{O}_{11}}\]
done
clear
D)
\[C{{H}_{3}}COOH\]
done
clear
View Answer play_arrow
question_answer 135) Which of the following is used during discovery of Calvin cycle?
A)
Spirogyra
done
clear
B)
Volvox
done
clear
C)
Chlamydomonas
done
clear
D)
Chlorella
done
clear
View Answer play_arrow
question_answer 136) Respiratory quotient of which diet is less than unity?
A)
Carbohydrate
done
clear
B)
Fats
done
clear
C)
Organic acids
done
clear
D)
Sugar
done
clear
View Answer play_arrow
question_answer 137) Velamen and spongy tissue is found in:
A)
breathing roots
done
clear
B)
parasitic
done
clear
C)
tuberous roots
done
clear
D)
epiphytic roots
done
clear
View Answer play_arrow
question_answer 138) The function of clitellum in Pheretima is:
A)
formation of cocoon
done
clear
B)
secretion of hormone
done
clear
C)
nutrition of sperm
done
clear
D)
respiration
done
clear
View Answer play_arrow
question_answer 139) Which of the following is not a function of water in cell?
A)
It provides energy for chemical reaction
done
clear
B)
It act as a solvent
done
clear
C)
It provides a medium for chemical reaction
done
clear
D)
It release hydrogen ions on ionization
done
clear
View Answer play_arrow
question_answer 140) In which of the following reptiles four chambered heart is present?
A)
Lizard
done
clear
B)
Snake
done
clear
C)
Scorpion
done
clear
D)
Crocodile
done
clear
View Answer play_arrow
question_answer 141) Rate of transpiration is measured by:
A)
nanometer
done
clear
B)
auxanometer
done
clear
C)
pofometer
done
clear
D)
barometer
done
clear
View Answer play_arrow
question_answer 142) Pigments present in chloroplast of Ulothrix:
A)
chlorophyll-a, chl. b, fucoxanthin \[\beta -\]carotene
done
clear
B)
chl. a, chl. b, chl. c, c-phycocyanin, c-phycoerythrin
done
clear
C)
chl. a, chl. b, \[\beta -\]carotene, xanthophyll
done
clear
D)
chl. a, chl. b, r-phycocyanin r-phycoerythrin
done
clear
View Answer play_arrow
question_answer 143) In Mirabilis a hybrid for red (RR) and white (rr) flower produces pink (Rr) flower. A plant with pink flower is crossed with white flower the expected phenotypic ratio is:
A)
red: pink : white (1 : 2:1)
done
clear
B)
pink: white : (1 : 1)
done
clear
C)
red: pink (1:1)
done
clear
D)
red: pink (3:1)
done
clear
View Answer play_arrow
question_answer 144) Which of the following is the site of lipid synthesis?
A)
Rough ER
done
clear
B)
Smooth ER
done
clear
C)
Golgi bodies
done
clear
D)
Ribosome
done
clear
View Answer play_arrow
question_answer 145) Which of the following vessel in rabbit starts with capillaries and ends in capillaries?
A)
Pulmonary artery
done
clear
B)
Renal vein
done
clear
C)
Hepatic portal vein
done
clear
D)
Renal artery
done
clear
View Answer play_arrow
question_answer 146) Which character is not same in aves and mammals?
A)
Single systemic arch
done
clear
B)
Metaneptiric kidney
done
clear
C)
Seven cervical vertebrae
done
clear
D)
Homiotherms
done
clear
View Answer play_arrow
question_answer 147) What is pollen grain?
A)
Microspore mother cell
done
clear
B)
Male gamete
done
clear
C)
Male gametophyte
done
clear
D)
Partially developed embryo
done
clear
View Answer play_arrow
question_answer 148) Solenocytes and nephridia are respectively found in:
A)
Platyhelminth and Annelids
done
clear
B)
Annelids and Nematoda
done
clear
C)
Cnidaria and Mollusca
done
clear
D)
Mollusca and Echinodermata
done
clear
View Answer play_arrow
question_answer 149) If a cell shrinks when placed in a solution, this solution is:
A)
hypotonic
done
clear
B)
hypertonic
done
clear
C)
isotonic
done
clear
D)
mesotonic
done
clear
View Answer play_arrow
question_answer 150) Aquatic reptiles are:
A)
ammonotelic
done
clear
B)
ureotelic
done
clear
C)
ureotelic in water
done
clear
D)
ureotelic over land
done
clear
View Answer play_arrow