question_answer 1) A long spring is stretched by 2 cm. Its potential energy is U. If the spring is stretched by 10 cm, the potential energy stored in it will be:
A)
\[\frac{U}{25}\]
done
clear
B)
\[\frac{U}{5}\]
done
clear
C)
\[3U\]
done
clear
D)
\[25U\]
done
clear
View Answer play_arrow
question_answer 2) The coefficient of restitution e for a perfectly elastic collision is:
A)
1
done
clear
B)
zero
done
clear
C)
infinite
done
clear
D)
\[-1\]
done
clear
View Answer play_arrow
question_answer 3) A soap bubble is given negative charge. Then its radius:
A)
decreases
done
clear
B)
increases
done
clear
C)
remains unchanged
done
clear
D)
nothing can be said because sufficient information is not available
done
clear
View Answer play_arrow
question_answer 4) Source of energy in sun is caused by :
A)
fusion of heavy nuclei
done
clear
B)
fission of heavy nuclei
done
clear
C)
fusion of hydrogen nuclei
done
clear
D)
fission of hydrogen nuclei
done
clear
View Answer play_arrow
question_answer 5) A particle moves along a circular path under the action of a force. The work done by the force is:
A)
positive and non-zero
done
clear
B)
negative and non-zero
done
clear
C)
zero
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 6) A particle is moving in a circle with uniform speed. It has constant:
A)
velocity
done
clear
B)
acceleration
done
clear
C)
kinetic energy
done
clear
D)
displacement
done
clear
View Answer play_arrow
question_answer 7) If \[|\vec{A}+\vec{B}|=|\vec{A}-\vec{B}|,A\]and B are finite then
A)
\[\overset{\to }{\mathop{A}}\,\text{ }is\text{ }parallel\text{ }to\text{ }\overset{\to }{\mathop{B}}\,\]
done
clear
B)
\[\overset{\to }{\mathop{A}}\,\text{ }is\text{ }anti-parallel\text{ }to\text{ }\overset{\to }{\mathop{B}}\,\]
done
clear
C)
\[\overset{\to }{\mathop{A}}\,\text{ }and\,\,\overset{\to }{\mathop{B}}\,\,are\,\,equal\,\,in\,\,magnitude\]
done
clear
D)
\[\overset{\to }{\mathop{A}}\,\text{ }and\overset{\to }{\mathop{B}}\,are\,\,mutually\,\,perpendicular\]
done
clear
View Answer play_arrow
question_answer 8) A number of small drops of mercury adiabatically coalesce, to form a single drop. The temperature of the drop:
A)
increases
done
clear
B)
remains same
done
clear
C)
decreases
done
clear
D)
depend on size
done
clear
View Answer play_arrow
question_answer 9) Light waves can be polarised as they are :
A)
transverse
done
clear
B)
of high frequency
done
clear
C)
longitudinal
done
clear
D)
reflected
done
clear
View Answer play_arrow
question_answer 10) In which process the speed of transfer of heat is maximum?
A)
Conduction
done
clear
B)
Convection
done
clear
C)
Radiation
done
clear
D)
In all these heat is transferred with the same speed
done
clear
View Answer play_arrow
question_answer 11) Youngs experiment establishes that:
A)
light consists of waves
done
clear
B)
light consists of particles
done
clear
C)
light is neither of particles nor waves
done
clear
D)
light consists of particles and waves both
done
clear
View Answer play_arrow
question_answer 12) A heating coil is labelled 100 W, 220V. The coil is cut into two equal pieces and the two pieces are joined in parallel to the same source. The energy now liberated per second is:
A)
400 J
done
clear
B)
25 J
done
clear
C)
50 J
done
clear
D)
200J
done
clear
View Answer play_arrow
question_answer 13) Bending of light rays around the edges of an obstacle is known as:
A)
refraction
done
clear
B)
polarisation
done
clear
C)
diffraction
done
clear
D)
reflection
done
clear
View Answer play_arrow
question_answer 14) A convex lens of focal length 40cm is in contact with a concave lens of focal length 25 cm. The power of the combination of the two lenses is:
A)
\[-1.5D\]
done
clear
B)
\[~-6.5\text{ }D\]
done
clear
C)
6.5 D
done
clear
D)
6.67 D
done
clear
View Answer play_arrow
question_answer 15) Particle A moves in S-N direction and particle B moves in W-E direction. Then the velocity-of L particle A with respect to B is:
A)
N-W direction
done
clear
B)
N-E direction
done
clear
C)
S-W direction
done
clear
D)
S-E direction
done
clear
View Answer play_arrow
question_answer 16) Weightlessness experienced while orbiting the earth in space ships is the result of:
A)
inertia
done
clear
B)
acceleration
done
clear
C)
zero gravity
done
clear
D)
centre of gravity
done
clear
View Answer play_arrow
question_answer 17) If one mole of a monoatomic gas \[(\gamma =5/3)\]is mixed with one mole of a diatomic gas \[(\gamma =7/5),\] the value of \[\gamma \]for the mixture is:
A)
1.40
done
clear
B)
1.50
done
clear
C)
1.53
done
clear
D)
3.07
done
clear
View Answer play_arrow
question_answer 18) Which of the following statements is wrong?
A)
Sound travels in straight line
done
clear
B)
Sound travels as waves
done
clear
C)
Sound is a form of energy
done
clear
D)
Sound travels faster in vacuum than in air
done
clear
View Answer play_arrow
question_answer 19) If momentum of a certain body is increased by 50%, then increases in the KE of the body will be:
A)
25%
done
clear
B)
50%
done
clear
C)
100%
done
clear
D)
125%
done
clear
View Answer play_arrow
question_answer 20) A uniform chain of length L and mass M is lying on a smooth table and one-third of its length is hanging vertically down over the edge of the table, if g is the acceleration due to gravity the work required to pull the hanging part of the chain on the table is:
A)
\[MgL\]
done
clear
B)
\[\frac{1}{3}MgL\]
done
clear
C)
\[\frac{1}{9}MgL\]
done
clear
D)
\[\frac{1}{18}MgL\]
done
clear
View Answer play_arrow
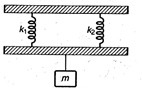
question_answer 21)
In the arrangement shown in the figure for vertical oscillations of the mass \[m,\]the period is:
A)
\[T=2\pi \sqrt{\frac{m}{{{k}_{1}}+{{k}_{2}}}}\]
done
clear
B)
\[T=2\pi \sqrt{\frac{{{k}_{1}}+{{k}_{2}}}{m}}\]
done
clear
C)
\[T=2\pi \sqrt{\frac{m({{k}_{1}}+{{k}_{2}})}{{{k}_{1}}+{{k}_{2}}}}\]
done
clear
D)
\[T=2\pi \sqrt{\frac{mg}{{{k}_{1}}+{{k}_{2}}}}\]
done
clear
View Answer play_arrow
question_answer 22) A person completes half of its journey with speed\[{{v}_{1}}\]and rest half with speed \[{{v}_{2}}.\]The average speed of the person is:
A)
\[v=\frac{1}{2}({{v}_{1}}+{{v}_{2}})\]
done
clear
B)
\[v=\frac{2{{v}_{1}}{{v}_{2}}}{{{v}_{1}}+{{v}_{2}}}\]
done
clear
C)
\[v=\frac{{{v}_{1}}{{v}_{2}}}{{{v}_{1}}+{{v}_{2}}}\]
done
clear
D)
\[v=\sqrt{{{v}_{1}}{{v}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 23) A particle moves along a straight line such that its displacement at any time t is given by \[s={{t}^{3}}-3{{t}^{2}}+2m.\]The displacement when the acceleration bec6mes zero is:
A)
zero
done
clear
B)
2 m
done
clear
C)
3m
done
clear
D)
-2m
done
clear
View Answer play_arrow
question_answer 24) \[[M{{L}^{2}}{{T}^{-3}}]\]represents the dimensions of:
A)
pressure
done
clear
B)
energy
done
clear
C)
power
done
clear
D)
force
done
clear
View Answer play_arrow
question_answer 25) The time period of a simple pendulum in a lift descending with constant acceleration g is:
A)
\[T=2\pi \sqrt{\frac{l}{8}}\]
done
clear
B)
\[T=2\pi \sqrt{\frac{l}{2g}}\]
done
clear
C)
zero
done
clear
D)
infinite
done
clear
View Answer play_arrow
question_answer 26) A transverse wave is given by \[y=A\sin 2\pi \left( \frac{t}{T}-\frac{x}{\lambda } \right).\] The maximum particle velocity is equal to 4 times the wave velocity, when:
A)
\[\lambda =2\pi \,A\]
done
clear
B)
\[\lambda =\frac{1}{2}\pi A\]
done
clear
C)
\[\lambda =\pi A\]
done
clear
D)
\[\lambda =\frac{1}{4}\pi A\]
done
clear
View Answer play_arrow
question_answer 27) A tuning fork gives 4 beats with 50 cm length of a sonometer wire. If the length of the wire is shortened by I cm the number of beats is still the same. The frequency of the fork is:
A)
396 Hz
done
clear
B)
400 Hz
done
clear
C)
404 Hz
done
clear
D)
384 Hz
done
clear
View Answer play_arrow
question_answer 28) Electron volt is a unit of:
A)
potential
done
clear
B)
charge
done
clear
C)
power
done
clear
D)
energy
done
clear
View Answer play_arrow
question_answer 29) Light of frequency,\[v\]is incident on a certain photoelectric substance with threshold frequency\[{{v}_{0}}.\]The work function for the substance is:
A)
\[hv\]
done
clear
B)
\[h{{v}_{0}}\]
done
clear
C)
\[h(v-{{v}_{0}})\]
done
clear
D)
\[h(v+{{v}_{0}})\]
done
clear
View Answer play_arrow
question_answer 30) For principal quantum number\[n=3,\]the possible values of orbital quantum number \[l\]are:
A)
1, 2, 3
done
clear
B)
0, 1, 2, 3
done
clear
C)
0, 1, 2
done
clear
D)
\[-1,0,+1\]
done
clear
View Answer play_arrow
question_answer 31) Certain radioactive substance reduces to 25% of its value in 16 days. Its half-life is:
A)
32 days
done
clear
B)
8 days
done
clear
C)
64 days
done
clear
D)
28 days
done
clear
View Answer play_arrow
question_answer 32) Penetrating power of X-rays does not depend on:
A)
wavelength
done
clear
B)
energy
done
clear
C)
potential difference
done
clear
D)
current in the filament
done
clear
View Answer play_arrow
question_answer 33) The ratio of resistance for forward to reverse bias of \[p-n\]junction is:
A)
\[{{10}^{2}}:1\]
done
clear
B)
\[{{10}^{-2}}:1\]
done
clear
C)
\[1:{{10}^{-4}}\]
done
clear
D)
\[1:{{10}^{4}}\]
done
clear
View Answer play_arrow
question_answer 34) If a current flows through an infinitely long straight wire, the magnetic field produced at a point 1m away from it, is:
A)
\[2\times {{10}^{-3}}T\]
done
clear
B)
\[2\times {{10}^{-1}}T\]
done
clear
C)
\[2\times {{10}^{-1}}T\]
done
clear
D)
\[2\pi \times {{10}^{-6}}T\]
done
clear
View Answer play_arrow
question_answer 35)
Two circular discs A and B with equal radii are blackened. They are heated to same temperature and are cooled under identical conditions. What inference do you draw from their cooling curves?
A)
A and B have same specific heats
done
clear
B)
Specific heat of A is less
done
clear
C)
Specific heat of B is less
done
clear
D)
Nothing can be said
done
clear
View Answer play_arrow
question_answer 36) When a charged particle, travelling with unifrom speed enters a uniform magnetic field perpendicularly then. Its kinetic energy:
A)
remains constant
done
clear
B)
increases
done
clear
C)
decreases
done
clear
D)
becomes zero
done
clear
View Answer play_arrow
question_answer 37) A circular coil of radius 4 cm and number of turns 20 carries a current of 3 A. It is placed in a magnetic field of 0.5 T. The magnetic dipole moment of the coil is:
A)
\[\text{0}\text{.60 A}{{\text{m}}^{\text{2}}}\]
done
clear
B)
\[~0.45\text{ A}{{\text{m}}^{2}}\]
done
clear
C)
\[\text{ }\!\!~\!\!\text{ 0}\text{.30 A}{{\text{m}}^{\text{2}}}\]
done
clear
D)
\[~0.15A{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 38) In a circuit 5% of total current passes through a galvanometer. If resistance of the galvanometer is G, then value of the shunt is:
A)
19 G
done
clear
B)
20 G
done
clear
C)
G/20
done
clear
D)
G/19
done
clear
View Answer play_arrow
question_answer 39) In a moving coil galvanometer, deflection \[\text{o }\!\!|\!\!\text{ }\] and current I flowing through it are related by:
A)
\[I\propto \tan \text{o }\!\!|\!\!\text{ }\]
done
clear
B)
\[I\propto \text{o }\!\!|\!\!\text{ }\]
done
clear
C)
\[I\propto \text{o}{{\text{ }\!\!|\!\!\text{ }}^{2}}\]
done
clear
D)
\[I\propto 1/\text{o }\!\!|\!\!\text{ }\]
done
clear
View Answer play_arrow
question_answer 40) A solenoid of length\[l\]metre has self-inductance L henry. If number of turns are doubled, its self inductance:
A)
remains same
done
clear
B)
becomes 2 L henry
done
clear
C)
becomes 4 L henry
done
clear
D)
becomes\[\frac{L}{\sqrt{2}}\] henry
done
clear
View Answer play_arrow
question_answer 41) The voltage of an AC supply varies with time (t) as \[V=120\sin 100\,\pi t\,\cos 100\,\pi t.\]The maximum voltage and frequency respectively are:
A)
\[120V,\text{ }100\text{ }Hz\]
done
clear
B)
\[120/\sqrt{2}\,V,100\,Hz\]
done
clear
C)
\[60\,V,\,200\,Hz\]
done
clear
D)
\[60\,V,\,100\,Hz\]
done
clear
View Answer play_arrow
question_answer 42) Two point charges of\[+\,3\mu C\] and \[-\,3\mu C\]are at a distance \[2\times {{10}^{-3}}\,m\]apart from each other. The electric field at a distance of 0.6 m from the dipole in broadside-on position is:
A)
\[150\,N{{C}^{-1}}\]
done
clear
B)
\[250\,N{{C}^{-1}}\]
done
clear
C)
\[60\,N{{C}^{-1}}\]
done
clear
D)
\[35\,N{{C}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 43) Two parallel large thin metal sheets have equal surface charge densities \[(\sigma =26.4\times {{10}^{-12}}C/{{m}^{2}})\]of opposite signs. The electric field between these sheets is:
A)
\[1.5\,N/C\]
done
clear
B)
\[1.5\times {{10}^{-10}}N/C\]
done
clear
C)
\[3\,N/C\]
done
clear
D)
\[3\times {{10}^{-10}}N/C\]
done
clear
View Answer play_arrow
question_answer 44) N identical spherical drops are charged to the same potential V. They combine to form a bigger drop. The potential of the big drop will be:
A)
\[V{{N}^{1/3}}\]
done
clear
B)
\[V{{N}^{2/3}}\]
done
clear
C)
\[V\]
done
clear
D)
\[VN\]
done
clear
View Answer play_arrow
question_answer 45) Three condensers of capacitance 2uF each are connected in series. The resultant capacitance is:
A)
\[6\mu F\]
done
clear
B)
\[3/2\,\mu F\]
done
clear
C)
\[2/3\,\mu F\]
done
clear
D)
\[5\,\mu F\]
done
clear
View Answer play_arrow
question_answer 46) Two electric bulbs rated \[{{P}_{1}}\]watt, V volt and \[{{P}_{2}}\]watt, V volt are connected in parallel and V volt supply is applied to them. The total power will be:
A)
\[{{P}_{1}}+{{P}_{2}}\]
done
clear
B)
\[\sqrt{{{P}_{1}}{{P}_{2}}}\]
done
clear
C)
\[\frac{{{P}_{1}}{{P}_{2}}}{{{P}_{1}}+{{P}_{2}}}\]
done
clear
D)
\[\frac{{{P}_{1}}+{{P}_{2}}}{{{P}_{1}}{{P}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 47) A hole is in the bottom of the tank having water. If total pressure at the bottom is 3 atm (\[(1\,atm={{10}^{5}}\,N{{m}^{-2}}),\]1 then velocity of water flowing from hole is:
A)
\[\sqrt{400}\,m{{s}^{-1}}\]
done
clear
B)
\[\sqrt{600}\,m{{s}^{-1}}\]
done
clear
C)
\[\sqrt{60}\,m{{s}^{-1}}\]
done
clear
D)
\[\sqrt{40}\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 48) A fluid of volume 1 L is subjected to a pressure change of \[{{10}^{7}}N/{{m}^{2}}.\] As a result its volume changes by \[0.4\,c{{m}^{3}}.\] The bulk modulus of the fluid is:
A)
\[2.5\times {{10}^{10}}\,N/{{m}^{2}}\]
done
clear
B)
\[2.5\times {{10}^{11}}\,N/{{m}^{2}}\]
done
clear
C)
\[2.5\times {{10}^{9}}\,N/{{m}^{2}}\]
done
clear
D)
\[2.5\times {{10}^{15}}\,N/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 49) Stationary waves are so called because in them:
A)
the particles of the medium are not disturbed at all
done
clear
B)
the particles of the medium do not execute SHM
done
clear
C)
Particles do not correspond flow of energy along the wire
done
clear
D)
the interference effect cannot be observed
done
clear
View Answer play_arrow
question_answer 50) If the degrees of freedom of the molecules of a gas are n, the ratio of its two specific heats \[({{C}_{P}}+{{C}_{V}})\]will be:
A)
\[1+\frac{2}{n}\]
done
clear
B)
\[1-\frac{2}{n}\]
done
clear
C)
\[1+\frac{1}{n}\]
done
clear
D)
\[2-\frac{1}{n}\]
done
clear
View Answer play_arrow
question_answer 51) In a radioactive decay:
A)
\[\alpha \] then\[\beta \] and then\[\gamma \]emitted
done
clear
B)
\[\alpha \] or \[\beta \] and then\[\gamma \]emitted
done
clear
C)
\[\alpha \]and\[\beta \] and \[\gamma \]emitted simultaneously
done
clear
D)
\[\alpha \]and\[\beta \]emitted simultaneously
done
clear
View Answer play_arrow
question_answer 52) The work function of a metal is 1 eV. If\[\text{3000}\,\overset{\text{o}}{\mathop{\text{A}}}\,\] wavelength light is incident, the value of stopping voltage is:
A)
1V
done
clear
B)
\[\text{3}\text{.75}\,\text{V}\]
done
clear
C)
3.2 V
done
clear
D)
0.75 V
done
clear
View Answer play_arrow
question_answer 53) Equation for a real gas is \[\left( P+\frac{a}{{{V}^{2}}} \right)(V-b)=RT\] Where\[P=\]pressure, \[V=\]volume, a, b and R constants, dimensional formula of a is:
A)
\[{{L}^{6}}\]
done
clear
B)
\[{{M}^{1}}{{L}^{-1}}{{T}^{-2}}\]
done
clear
C)
\[{{M}^{1}}{{L}^{5}}{{T}^{-2}}\]
done
clear
D)
\[{{L}^{3}}\]
done
clear
View Answer play_arrow
question_answer 54) A monoatomic ideal gas is compressed to its 1/8 volume adiabatically at \[17{{\,}^{o}}C.\] Temperature after compression will tie:
A)
\[34{{\,}^{o}}C\]
done
clear
B)
\[17{{\,}^{o}}C\]
done
clear
C)
\[136{{\,}^{o}}C\]
done
clear
D)
\[887{{\,}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 55) Which quantity remains constant in adiabatic process?
A)
Work
done
clear
B)
Heat
done
clear
C)
Temperature
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 56) Which formula is incorrect for root mea square velocity?
A)
\[\sqrt{\frac{2RT}{M}}\]
done
clear
B)
\[\sqrt{\frac{3PV}{M}}\]
done
clear
C)
\[\sqrt{\frac{3d}{P}}\]
done
clear
D)
\[\sqrt{\frac{2KE}{M}}\]
done
clear
View Answer play_arrow
question_answer 57) If the number of molecules of hydrogen is double to that of oxygen, at the same temperature, the ratio of their average KE pa molecule is:
A)
\[1:1\]
done
clear
B)
\[2:3\]
done
clear
C)
\[1:2\]
done
clear
D)
\[1:4\]
done
clear
View Answer play_arrow
question_answer 58) A liquid boils at that temperature at which the pressure of saturated vapour is:
A)
more than atmospheric pressure
done
clear
B)
double to atmospheric pressure
done
clear
C)
equal to atmospheric pressure
done
clear
D)
less than atmospheric pressure
done
clear
View Answer play_arrow
question_answer 59) What is the value of \[\frac{PV}{T}\] for a 1 mote of gas?
A)
\[4.2\times {{10}^{7}}cal/K\]
done
clear
B)
\[4.2\,cal/K\]
done
clear
C)
\[8.31\,cal/K\]
done
clear
D)
\[2\,cal/K\]
done
clear
View Answer play_arrow
question_answer 60) If a proton totally converts into energy, the value of energy will be:
A)
190 MeV
done
clear
B)
931 MeV
done
clear
C)
93.1 MeV
done
clear
D)
931 J
done
clear
View Answer play_arrow
question_answer 61) Experimental verification of matter waves was done by:
A)
de-Broglie
done
clear
B)
Rutherford
done
clear
C)
Bohr
done
clear
D)
Davisson and Germer
done
clear
View Answer play_arrow
question_answer 62) In the following nuclear reaction \[_{2}H{{e}^{3}}+{{\,}_{Z}}{{X}^{a}}\xrightarrow{{}}{{\,}_{Z+1}}{{Y}^{a+2}}+Q.\]What is Q?
A)
Neutron
done
clear
B)
Proton
done
clear
C)
Positron
done
clear
D)
Electron
done
clear
View Answer play_arrow
question_answer 63) For the reaction \[{{\,}_{1}}{{H}^{2}}+{{\,}_{1}}{{H}^{2}}\xrightarrow{{}}{{\,}_{2}}H{{e}^{4}}+v+\text{Energy,}\] what is the required condition?
A)
High temperature and pressure
done
clear
B)
High temperature and low pressure
done
clear
C)
Low temperature and high pressure
done
clear
D)
High temperature only
done
clear
View Answer play_arrow
question_answer 64) Work function of photoelectric metal is 3.13 eV. Threshold frequency is:
A)
\[4\times {{10}^{11}}\,Hz\]
done
clear
B)
\[5\times {{10}^{14}}\,Hz\]
done
clear
C)
\[8\times {{10}^{15}}\,Hz\]
done
clear
D)
\[8\times {{10}^{10}}\,Hz\]
done
clear
View Answer play_arrow
question_answer 65) Nitro group of nitrobenzene is:
A)
\[o-\]directing
done
clear
B)
\[m-\] direcdng
done
clear
C)
\[p-\]directing
done
clear
D)
o, and p directing
done
clear
View Answer play_arrow
question_answer 66) In which compound the number of 3° carbon is maximum:
A)
2, 5 dimethyl hexane
done
clear
B)
2, 3, 4-trimethyl pentane
done
clear
C)
2, 2, 4, 4-tetramethyl pentane
done
clear
D)
2, 2, 3, trimethyl pentane
done
clear
View Answer play_arrow
question_answer 67) The pH of a solution of concentration few less than 1 N NaOH:
A)
between 13 and 14
done
clear
B)
between 12 and 13
done
clear
C)
between 0 and 1
done
clear
D)
between 1 and 2
done
clear
View Answer play_arrow
question_answer 68) Dichlorocarbene is:
A)
a neutral divalent species
done
clear
B)
a carbonation
done
clear
C)
a carbanion
done
clear
D)
a free radical
done
clear
View Answer play_arrow
question_answer 69) The geometry of the hybrid orbital, which contains 20% s-character is:
A)
octahedral
done
clear
B)
tetrahedral
done
clear
C)
trigonal bipyramidal
done
clear
D)
square planar
done
clear
View Answer play_arrow
question_answer 70) Which test is not ideal to distinguish 2-butanol and 1-propanol?
A)
Hydrogenation
done
clear
B)
lodoform test
done
clear
C)
Lucas test
done
clear
D)
Oxidation test
done
clear
View Answer play_arrow
question_answer 71) In the reaction of\[C{{H}_{2}}=C{{H}_{2}}\]and HBr initial addition occurs of:
A)
indefinite
done
clear
B)
\[{{H}^{+}}+B{{r}^{-}}\]both at one time
done
clear
C)
\[{{H}^{+}}\]
done
clear
D)
\[B{{r}^{-}}\]
done
clear
View Answer play_arrow
question_answer 72) The hydrocarbon formed by electrolysis of sodium propionate:
A)
\[C{{H}_{3}}CH=C{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 73) 0.1M\[C{{H}_{3}}COOH\]is 1.3% ionised. The dissociation constant of it will be:
A)
\[1.69\times {{10}^{-5}}\]
done
clear
B)
\[1.69\times {{10}^{-6}}\]
done
clear
C)
\[1.69\times {{10}^{-4}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 74) Which of the following give dye test?
A)
Aniline
done
clear
B)
Methylamine
done
clear
C)
Diphenylamine
done
clear
D)
Ethylamine
done
clear
View Answer play_arrow
question_answer 75) When ethanamide is heated with NaOH and \[B{{r}_{2}}\]the compound formed is:
A)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}NC\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 76) The chemical formula of potash alum is \[{{K}_{2}}S{{O}_{4}}.A{{l}_{2}}{{(S{{O}_{4}})}_{3}}x{{H}_{2}}O,\]here \[x\] is:
A)
7
done
clear
B)
12
done
clear
C)
6
done
clear
D)
24
done
clear
View Answer play_arrow
question_answer 77) Brass is an alloy of:
A)
\[Cu+Zn+Fe\]
done
clear
B)
\[Cu+Zn+Ni\]
done
clear
C)
\[Cu+Zn+Sn\]
done
clear
D)
\[Cu+Zn\]
done
clear
View Answer play_arrow
question_answer 78) The electronic configuration of \[F{{e}_{26}}\]is [Ar]:
A)
\[~3{{d}^{8}}4{{s}^{2}}\]
done
clear
B)
\[~3{{d}^{7}}\text{ }4{{s}^{2}}\]
done
clear
C)
\[~3{{d}^{6}}4{{s}^{2}}\]
done
clear
D)
\[~3{{d}^{5}}4{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 79) The rate constant of forward reaction is \[2.38\times {{10}^{-4}}\]and the rate constant of backward reaction is \[4.76\times {{10}^{-5}}.\] The equilibrium constant for the reaction will be:
A)
5
done
clear
B)
\[5\times {{10}^{-2}}\]
done
clear
C)
\[2\times {{10}^{-4}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 80) The element with electronic configuration \[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}},4{{s}^{2}}\]shows same property as:
A)
Mo
done
clear
B)
Rb
done
clear
C)
Ca
done
clear
D)
Sr
done
clear
View Answer play_arrow
question_answer 81) How much volume of 0.4 M NaOH is required to neutralise completely 200 mL \[\text{0}\text{.5}\,\text{M}\,{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]solution?
A)
600 mL
done
clear
B)
300 mL
done
clear
C)
500 mL
done
clear
D)
200 Ml
done
clear
View Answer play_arrow
question_answer 82) The formula of acetaldehyde semicarbazone:
A)
\[C{{H}_{3}}-CH=NNHCONHC{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-CH=N-OH\]
done
clear
C)
\[C{{H}_{3}}CH=N-NHCON{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}-CH=N-NHCONH-CON{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 83) One compound reacts with chloroform in presence of KOH and produces a bad smell (nause odour) compound. The compound formed is:
A)
\[C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}CN\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}NC\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}CN\]
done
clear
View Answer play_arrow
question_answer 84) Phenol, chloroform and caustic potash are heated. The compound formed is:
A)
salicylic acid
done
clear
B)
\[p-\] hydroxy benzaldehyde
done
clear
C)
\[m-\] hydroxy benzaldehyde
done
clear
D)
salicylaldehyde
done
clear
View Answer play_arrow
question_answer 85) \[A+NaOH\xrightarrow{{}}C{{H}_{3}}OH+HCOONa\]is the reaction, compound A is:
A)
HCN
done
clear
B)
HCHO
done
clear
C)
\[C{{H}_{3}}CN\]
done
clear
D)
\[C{{H}_{3}}Cl\]
done
clear
View Answer play_arrow
question_answer 86) The compound obtained by the reaction of acetic anhydride and ammonia is:
A)
\[C{{H}_{3}}COON{{H}_{4}}\]
done
clear
B)
\[~C{{H}_{3}}CN\]
done
clear
C)
\[~C{{H}_{3}}CONHC{{H}_{3}}\]
done
clear
D)
\[~C{{H}_{3}}CON{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 87) Metal which does not react with aqueous solution of copper sulphate is:
A)
Pb
done
clear
B)
Ag
done
clear
C)
Zn
done
clear
D)
Fe
done
clear
View Answer play_arrow
question_answer 88) When aniline react with acetic anhydride the product formed is:
A)
\[p-\]aminobenzoic acid
done
clear
B)
\[m-\]aminobenzoic acid
done
clear
C)
acetanilide
done
clear
D)
\[o-\]aminobenzoic acid
done
clear
View Answer play_arrow
question_answer 89) \[5SO_{2}^{-}+2MnO_{4}^{-}+6{{H}^{+}}\xrightarrow{{}}5SO_{4}^{2-}\]\[+\,2M{{n}^{2+}}+2{{H}_{2}}O\] the oxidation number of Mn changes from:
A)
\[+\,14\,\text{to}+4\]
done
clear
B)
\[+\,6\,\text{to}+2\]
done
clear
C)
\[-7\,\text{to}-2\]
done
clear
D)
\[+7\,\text{to}+2\]
done
clear
View Answer play_arrow
question_answer 90) Which has highest ionisation potential?
A)
N
done
clear
B)
O
done
clear
C)
\[{{O}^{+}}\]
done
clear
D)
Na
done
clear
View Answer play_arrow
question_answer 91) Formic acid and acetic acid may be distinguished by the reaction with:
A)
sodium
done
clear
B)
2, 4-dinitrophenyl hydrazine
done
clear
C)
litmus paper
done
clear
D)
Tollens reagent
done
clear
View Answer play_arrow
question_answer 92) Maximum melting point is of:
A)
\[~MgC{{l}_{2}}\]
done
clear
B)
\[~BaC{{l}_{2}}~\]
done
clear
C)
\[~CaC{{l}_{2}}\]
done
clear
D)
\[~BeC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 93) The process requiring the absorption of energy is:
A)
\[F\xrightarrow{{}}{{F}^{-}}\]
done
clear
B)
\[Cl-C{{l}^{-}}\]
done
clear
C)
\[O\xrightarrow{{}}{{O}^{2-}}\]
done
clear
D)
\[H\xrightarrow{{}}{{H}^{-}}\]
done
clear
View Answer play_arrow
question_answer 94) The number of coordinate bond in a molecule of \[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{:}\]
A)
4
done
clear
B)
3
done
clear
C)
2
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 95) Camallite is:
A)
\[KCl\]
done
clear
B)
\[LiAl{{(Si{{O}_{3}})}_{2}}\]
done
clear
C)
\[MgC{{l}_{2}}.6{{H}_{2}}O\]
done
clear
D)
\[KCl.MgC{{l}_{2}}.6{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 96) A piece of magnesium ribbon was heated to redness in an atmosphere of nitrogen and on cooling water was added. The gas evolved was:
A)
ammonia
done
clear
B)
hydrogen
done
clear
C)
nitrogen
done
clear
D)
oxygen
done
clear
View Answer play_arrow
question_answer 97) Which of the following halides is least stable and doubtful existence?
A)
\[C{{l}_{4}}\]
done
clear
B)
\[Sn{{l}_{4}}\]
done
clear
C)
\[Ge{{l}_{4}}\]
done
clear
D)
\[Pb{{l}_{4}}\]
done
clear
View Answer play_arrow
question_answer 98) Lithopone is a mixture of:
A)
\[ZnS\]and \[{{K}_{2}}S{{O}_{4}}\]
done
clear
B)
\[CaC{{l}_{2}}\]and \[C{{a}_{3}}{{F}_{2}}\]
done
clear
C)
\[Ca{{C}_{2}}\]and \[C{{a}_{3}}{{N}_{2}}\]
done
clear
D)
\[ZnS+BaS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 99) Iodine is liberated from KI solution when treated with:
A)
\[ZnS{{O}_{4}}\]
done
clear
B)
\[CuS{{O}_{4}}\]
done
clear
C)
\[NiS{{O}_{4}}\]
done
clear
D)
\[FeS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 100) Chlorine acts as a bleaching agent only in the presence of:
A)
dry air
done
clear
B)
sun light
done
clear
C)
moisture
done
clear
D)
pure oxygen
done
clear
View Answer play_arrow
question_answer 101) Photoperiodic response of red and far-red wavelength is mediated by:
A)
phytochrome
done
clear
B)
cytochrome
done
clear
C)
ferredoxin
done
clear
D)
plastocyanin
done
clear
View Answer play_arrow
question_answer 102) Tissue that contains fat globules is known as:
A)
duraepithelium
done
clear
B)
adipose tissue
done
clear
C)
nepiepithelium
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 103) Which of the following inactivates the enzyme by changing its shape?
A)
Competitive inhibitor
done
clear
B)
Allosteric enzyme
done
clear
C)
Co-enzyme
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 104) Freshly hatched larva of housefly is called :
A)
caterpillar
done
clear
B)
maggot
done
clear
C)
nymph
done
clear
D)
grub
done
clear
View Answer play_arrow
question_answer 105) In human foetus, the heart begins to beat at developmental age of:
A)
4th week
done
clear
B)
3rd week
done
clear
C)
6th week
done
clear
D)
8th week
done
clear
View Answer play_arrow
question_answer 106) Enzymes that catalyse endergonic synthesis, coupled with exergonic hydrolysis of ATP are:
A)
ligases
done
clear
B)
isomerase
done
clear
C)
lyases
done
clear
D)
transferases
done
clear
View Answer play_arrow
question_answer 107) Gas exchange in the gills of a teleost fish is enhanced by having the blood flow in direction opposite to the direction of water flow, a process known as:
A)
counter current exchange
done
clear
B)
ventilation
done
clear
C)
facilitated diffusion
done
clear
D)
active respiration
done
clear
View Answer play_arrow
question_answer 108) Parkinsons disease is associated with:
A)
midbrain
done
clear
B)
thalamus
done
clear
C)
hypbthalamus
done
clear
D)
cerebrum
done
clear
View Answer play_arrow
question_answer 109) The branch of science dealing with the study of congenitally deformed foetus is called:
A)
teratology
done
clear
B)
gerontology
done
clear
C)
nidology
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 110) Sub-kingdom-Parazoa includes:
A)
Protozoa
done
clear
B)
Porifera
done
clear
C)
Metazoa
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 111) Tears contain which enzyme that act as antiseptic:
A)
muramidase
done
clear
B)
zymozyme
done
clear
C)
phagozyme
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 112) Organisms that are unable to tolerate wide variations of salt concentrations in the environment are termed:
A)
stenohaline
done
clear
B)
euryhaline
done
clear
C)
enohaline
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 113) Non-essential amino acids, are synthesized in the human body during:
A)
protein catabolism
done
clear
B)
protein anabolism
done
clear
C)
both [a] and [b]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 114) Sharpeys perforating fibres are related with:
A)
contraction of muscles
done
clear
B)
relaxation of muscles
done
clear
C)
fixing of teeth
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 115) Free martin is an example of:
A)
hormonal controlled unlike sexed twins
done
clear
B)
sex reversal by gene
done
clear
C)
environmental control of sex
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 116) Which of the following curve shows, how oxygen is loaded and unloaded due to partial pressure?
A)
\[{{\text{O}}_{\text{2}}}\]curve
done
clear
B)
\[C{{O}_{2}}\] curve
done
clear
C)
Bohrs curve
done
clear
D)
\[{{\text{O}}_{\text{2}}}\] dissociation curve
done
clear
View Answer play_arrow
question_answer 117) The polio virus enters the human body through:
A)
mosquito bite
done
clear
B)
tick bite
done
clear
C)
saliva and secretions from the nose
done
clear
D)
contaminated food and water
done
clear
View Answer play_arrow
question_answer 118) When there is no change in muscle length during contraction, it is called:
A)
isometric
done
clear
B)
isotonic
done
clear
C)
isostasis
done
clear
D)
refractory period
done
clear
View Answer play_arrow
question_answer 119) Which of the following organ has papillary muscles?
A)
Eyes
done
clear
B)
Brain
done
clear
C)
Heart
done
clear
D)
Mammary glands
done
clear
View Answer play_arrow
question_answer 120) The external and internal parts of cerebrospinal fluid are connected through The roof of:
A)
epithalamus
done
clear
B)
filum terminate
done
clear
C)
cerebral ventricles
done
clear
D)
medula oblongata
done
clear
View Answer play_arrow
question_answer 121) Vasopressin influences:
A)
electrolyte efflux
done
clear
B)
nerve excitability
done
clear
C)
water reabsorption
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 122) Widal test is performed for:
A)
malarial fever
done
clear
B)
cholera
done
clear
C)
typhoid fever
done
clear
D)
dengue fever
done
clear
View Answer play_arrow
question_answer 123) Pheromones when secreted upon the skin surface, its odour generally affects:
A)
skin colour
done
clear
B)
genitalia
done
clear
C)
breast
done
clear
D)
mutual behaviour of members of a species
done
clear
View Answer play_arrow
question_answer 124) Which response Amoeba shows towards current of water?
A)
Galvanotaxis
done
clear
B)
Rheotaxis
done
clear
C)
Geotaxis
done
clear
D)
Thigmotaxis
done
clear
View Answer play_arrow
question_answer 125) When more than one species of Plasmodium infect a person, it is called:
A)
quartan malaria
done
clear
B)
tertian malaria
done
clear
C)
aestivo- autumnal malaria
done
clear
D)
quotidian malaria
done
clear
View Answer play_arrow
question_answer 126) In which species of Paramecium, autogamy is found?
A)
Paramecium caudatum
done
clear
B)
Paramecium Aurelia
done
clear
C)
Paramecium furs aria
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 127) Coats disease is related with:
A)
eye
done
clear
B)
liver
done
clear
C)
brain
done
clear
D)
pancreas
done
clear
View Answer play_arrow
question_answer 128) The study of the energy transfer and relationships between all living organisms is known as:
A)
thermodynamics
done
clear
B)
bioenergetics
done
clear
C)
kinetic energy
done
clear
D)
potential energy
done
clear
View Answer play_arrow
question_answer 129) The infective stage ofFasciola hepatica to sheep is
A)
sporocyst
done
clear
B)
cercaria
done
clear
C)
metacercaria
done
clear
D)
hexacanth
done
clear
View Answer play_arrow
question_answer 130) Which of the following algae shows physiological anisogamy?
A)
Spirogyra
done
clear
B)
Ulothrix
done
clear
C)
Volvox
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 131) Rate of mutation is affected by:
A)
temperature
done
clear
B)
X-rays
done
clear
C)
gamma and beta radiations
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 132) A parenchymatous cell which stores ergostic materials or waste substance is:
A)
idioblast
done
clear
B)
phragmoblast
done
clear
C)
blastomere
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 133) Which of the following is applied for the biological control of cotton scale insect pest?
A)
Rhodalia cardinalis
done
clear
B)
Aleurolobus burodensis
done
clear
C)
Pectinophora gossypila
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 134) Which of the following is not a characteristics of the anatomy of dicotyledonous roots?
A)
Presence of little pith or absent
done
clear
B)
Radial vascular bundles
done
clear
C)
Presence of 15-20 vascular bundles
done
clear
D)
Secondary growth
done
clear
View Answer play_arrow
question_answer 135) The number of male prothallial cells in Selaginella plant is:
A)
one
done
clear
B)
two
done
clear
C)
twelve
done
clear
D)
numerous
done
clear
View Answer play_arrow
question_answer 136) Yeast is different from Penicillium and Rhizopus in being:
A)
acellular
done
clear
B)
unicellular
done
clear
C)
having unseptate hyphae
done
clear
D)
multicellular
done
clear
View Answer play_arrow
question_answer 137) In the \[{{\text{F}}_{\text{2}}}\]generation, genotypic and phenotypic ratios are identical in case of:
A)
complementary genes
done
clear
B)
Mendelian dihybrids
done
clear
C)
Mendelian monohybrids
done
clear
D)
incomplete dominance
done
clear
View Answer play_arrow
question_answer 138) In Funaria, the number of peristomial teeth is:
A)
8
done
clear
B)
16
done
clear
C)
32
done
clear
D)
64
done
clear
View Answer play_arrow
question_answer 139) The niche of a population is the:
A)
set of conditions that interacts
done
clear
B)
place where it lives
done
clear
C)
set of conditions and resources it uses
done
clear
D)
geographical area that it covers
done
clear
View Answer play_arrow
question_answer 140) Periblem is gives rise to:
A)
epidermis
done
clear
B)
cortex
done
clear
C)
pericycle
done
clear
D)
pith
done
clear
View Answer play_arrow
question_answer 141) A test-cross distinguishes between:
A)
a homozygous dominant and the heterozygous form
done
clear
B)
a homozygous recessive and the heterozygous form
done
clear
C)
two homozygous form
done
clear
D)
two heterozygous form
done
clear
View Answer play_arrow
question_answer 142) According to modern colloidal theory, the protoplasm is a polyphasic colloidal system. This was first suggested by:
A)
Purkinje
done
clear
B)
Max Schultz
done
clear
C)
R.A. Fisher
done
clear
D)
E. Strasburger
done
clear
View Answer play_arrow
question_answer 143) The scientific name of oyster mushroom, an edible fungus is:
A)
Tolyposporium penicillarie
done
clear
B)
Colletotrichum falcatum
done
clear
C)
Pleurotus osteratus
done
clear
D)
Citromyces purpurogenum
done
clear
View Answer play_arrow
question_answer 144) Verticillaster inflorescence is the characteristic of family:
A)
Cucurbitaceae
done
clear
B)
Rubiaceae
done
clear
C)
Labiatae
done
clear
D)
Asteraceae
done
clear
View Answer play_arrow
question_answer 145) In cyathium inflorescence, the ratio between male: female flower is:
A)
one : one
done
clear
B)
one : many
done
clear
C)
many : one
done
clear
D)
many : many
done
clear
View Answer play_arrow
question_answer 146) If a cell A with DPD 4 bars is connected to cell B, C, D whose Osmotic Pressure (OP) and Turgor Pressure (TP) are respectively 4 and 4, 10 and 5, 7 and 3 bars, the flow of water will be:
A)
B to A, C and D
done
clear
B)
A and D to B and C
done
clear
C)
C to A, B and D
done
clear
D)
A to B, C and D
done
clear
View Answer play_arrow
question_answer 147) Which one of the following is not a synthetic auxin?
A)
2, 4-D
done
clear
B)
IBA
done
clear
C)
IPA
done
clear
D)
IAA
done
clear
View Answer play_arrow
question_answer 148) The usual shape of growth curve is:
A)
sigmoid
done
clear
B)
zig-zag
done
clear
C)
linear
done
clear
D)
inverted bell-shaped
done
clear
View Answer play_arrow
question_answer 149) The beginning of plant cultivation is considered to be taken place in:
A)
Neolithic age
done
clear
B)
Paleolithic age
done
clear
C)
Mesolithic age
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 150) Which of the following groups is not comprised of sac-fungi?
A)
Yeasts
done
clear
B)
Truffles
done
clear
C)
Mushrooms
done
clear
D)
Morels
done
clear
View Answer play_arrow