question_answer 1) The twinkling effect of star light is due to
A)
total internal reflection
done
clear
B)
high dense matter of star
done
clear
C)
constant burning of hydrogen in the star
done
clear
D)
the fluctuating apparent position of the star being slightly different from the actual position of the star
done
clear
View Answer play_arrow
question_answer 2) The width of the diffraction band varies
A)
inversely as the wavelength
done
clear
B)
directly as the width of the slit
done
clear
C)
directly as the distance between the slit and the screen
done
clear
D)
inversely as the size of the source from which the slit is illuminated
done
clear
View Answer play_arrow
question_answer 3) An unpolarised beam of intensity \[{{I}_{0}}\] is incident on a pair of Nichols making an angle of \[{{60}^{o}}\] with each other. The intensity of light emerging from the pair is
A)
\[{{I}_{0}}\]
done
clear
B)
\[\frac{{{I}_{0}}}{2}\]
done
clear
C)
\[\frac{{{I}_{0}}}{4}\]
done
clear
D)
\[\frac{{{I}_{0}}}{8}\]
done
clear
View Answer play_arrow
question_answer 4) When a low flying aircraft passes over head, we sometimes notice a slight shaking of the picture on our TV screen. This is due to
A)
diffraction of the signal received from the antenna
done
clear
B)
interference of the direct signal received by the antenna with the weak signal reflected by the passing aircraft
done
clear
C)
change of magnetic flux occurring due to the passage of aircraft
done
clear
D)
vibration created by the, passage of aircraft,
done
clear
View Answer play_arrow
question_answer 5) A beam of light of wavelength \[600\,\,nm\] from a distant source falls on a single slit \[1\,\,mm\] wide and the resulting diffraction pattern is observed on a screen \[2\,\,m\] away. The distance between the first dark fringes on either side of the central bright fringe is
A)
\[1.2\,\,cm\]
done
clear
B)
\[1.2\,\,mm\]
done
clear
C)
\[2.4\,\,cm\]
done
clear
D)
\[2.4\,\,mm\]
done
clear
View Answer play_arrow
question_answer 6) The physical quantity having the dimensions \[[{{M}^{-1}}{{L}^{-3}}{{T}^{3}}{{A}^{2}}]\]is
A)
resistance
done
clear
B)
resistivity
done
clear
C)
electrical conductivity
done
clear
D)
electromotive force
done
clear
View Answer play_arrow
question_answer 7) A battery of emf \[10\,\,V\] and internal resistance \[3\Omega \] is connected to a resistor. The current in the circuit is\[0.5\,\,A\] The terminal voltage of the battery when the circuit is closed is
A)
\[10\,\,V\]
done
clear
B)
\[0\,\,V\]
done
clear
C)
\[1.5\,\,V\]
done
clear
D)
\[8.5\,\,V\]
done
clear
View Answer play_arrow
question_answer 8) A galvanometer coil has a resistance of \[15\Omega \] and gives full scale deflection for a current of\[4\,\,mA\]. To convert it to an am metal of range \[0\] to\[6\,\,A\]
A)
\[10\,\,m\Omega \] resistance is to be connected in parallel to the galvanometer
done
clear
B)
\[10\,\,m\Omega \] resistance is to be connected in series with the galvanometer
done
clear
C)
\[0.1\,\,\Omega \] resistance is to be connected in parallel to the galvanometer
done
clear
D)
\[0.1\,\,\Omega \] resistance is to be connected in series with the galvanometer
done
clear
View Answer play_arrow
question_answer 9) The electron drift speed is small and the charge of the electron is also small but still, we obtain large current in a conductor. This is due to
A)
the conducting property of the conductor
done
clear
B)
the resistance of the conductor is small
done
clear
C)
the electron number density of the conductor is small
done
clear
D)
the electron number density of the conductor is enormous
done
clear
View Answer play_arrow
question_answer 10) A straight wire of mass \[200\,\,g\] and length \[1.5\,\,m\] carries a current of\[2\,\,A\]. It is suspended in mid- air by a uniform horizontal magnetic field\[B\]. The magnitude of \[B\] (in tesla) is (assume\[g=9.8\,\,m{{s}^{-2}})\]
A)
\[2\]
done
clear
B)
\[1.5\]
done
clear
C)
\[0.55\]
done
clear
D)
\[0.65\]
done
clear
View Answer play_arrow
question_answer 11) A Gaussian sphere encloses an electric dipole within it. The total flux across the sphere is
A)
zero
done
clear
B)
half that due to a single charge
done
clear
C)
double that due to a single charge
done
clear
D)
dependent on the position of the dipole
done
clear
View Answer play_arrow
question_answer 12) A parallel plate air capacitor has a capacitance\[C\]. When it is half filled with a dielectric of dielectric constant\[5\], the percentage increase in the capacitance will be
A)
\[400%\]
done
clear
B)
\[66.6%\]
done
clear
C)
\[33.3%\]
done
clear
D)
\[200%\]
done
clear
View Answer play_arrow
question_answer 13) A comb run through one's dry hair attracts small bits of paper. This is due to
A)
comb is a good conductor
done
clear
B)
paper is a good conductor
done
clear
C)
the atoms is the paper get polarised by the charged comb
done
clear
D)
the comb possesses magnetic properties
done
clear
View Answer play_arrow
question_answer 14) The specific charge of a proton is\[9.6\times {{10}^{7}}C\,\,k{{g}^{-1}}\]. The specific charge of an alpha particle will be
A)
\[9.6\times {{10}^{7}}C\,\,k{{g}^{-1}}\]
done
clear
B)
\[19.2\times {{10}^{7}}C\,\,k{{g}^{-1}}\]
done
clear
C)
\[4.8\times {{10}^{7}}C\,\,k{{g}^{-1}}\]
done
clear
D)
\[2.4\times {{10}^{7}}C\,\,k{{g}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 15) When light of wavelength \[300\,\,nm\] falls on a photoelectric emitter, photoelectrons are liberated. For another emitter, light of wavelength \[600\,\,nm\] is sufficient for liberating photoelectrons. The ratio of the work function of the two emitters is
A)
\[1:2\]
done
clear
B)
\[2:1\]
done
clear
C)
\[4:1\]
done
clear
D)
\[1:4\]
done
clear
View Answer play_arrow
question_answer 16) White light is passed through a dilute solution of potassium permanganate. The spectrum produced by the emergent light is
A)
band emission spectrum
done
clear
B)
line emission spectrum
done
clear
C)
band absorption spectrum
done
clear
D)
line absorption spectrum
done
clear
View Answer play_arrow
question_answer 17) If \[{{\lambda }_{1}}\] and \[{{\lambda }_{2}}\] are the wavelengths of the first members of the Lyman and Paschen series respectively, then \[{{\lambda }_{1}}:{{\lambda }_{2}}\] is
A)
\[1:3\]
done
clear
B)
\[1:30\]
done
clear
C)
\[7:50\]
done
clear
D)
\[7:108\]
done
clear
View Answer play_arrow
question_answer 18) Activity of a radioactive sample decreases to \[{{(1/3)}^{rd}}\] of its original value in \[3\] days. Then, in \[9\] days its activity will become
A)
\[(1/27)\] of the original value
done
clear
B)
\[(1/9)\] of the original value
done
clear
C)
\[(1/18)\] of the original value
done
clear
D)
\[(1/3)\]of the original value
done
clear
View Answer play_arrow
question_answer 19) The working of which of the following is similar to that of a slide projector?
A)
Electron microscope
done
clear
B)
Scanning electron microscope
done
clear
C)
Transmission electron microscope
done
clear
D)
Atomic force microscope
done
clear
View Answer play_arrow
question_answer 20) In a transistor the collector current is always less than the emitter current because
A)
collector side is reverse biased and the emitter side is forward biased
done
clear
B)
a few electrons are lost in the base and only remaining ones reach the collector
done
clear
C)
collector being reverse biased, attracts less electrons
done
clear
D)
collector side is forward biased and emitter side is reverse biased
done
clear
View Answer play_arrow
question_answer 21) A transparent cube of \[0.21\,\,m\] edge contains a small air bubble. Its apparent distance when viewed through one face of the cube is \[0.10\,\,m\] and when viewed from the opposite face is\[0.04\,\,m\]. The actual distance of the bubble from the second face of the cube is
A)
\[0.06\,\,m\]
done
clear
B)
\[0.17\,\,m\]
done
clear
C)
\[0.05\,\,m\]
done
clear
D)
\[0.04\,\,m\]
done
clear
View Answer play_arrow
question_answer 22) To a fish under water, viewing obliquely a fisherman standing on the bank of a lake, the man looks
A)
taller than what he actually is
done
clear
B)
shorter that what he actually is
done
clear
C)
the same height as he actually is
done
clear
D)
depends on the obliquity
done
clear
View Answer play_arrow
question_answer 23) If white light is used in the Newton's rings experiment, the colour observed in the reflected light is complementary to that observed in the transmitted light through the same point. This is due to
A)
\[{{90}^{o}}\] change of phase in one of the reflected waves
done
clear
B)
\[{{180}^{o}}\] change of phase in one of the reflected waves
done
clear
C)
\[{{145}^{o}}\] change of phase in one of the reflected waves
done
clear
D)
\[{{45}^{o}}\] change of phase in one the reflected waves
done
clear
View Answer play_arrow
question_answer 24) A satellite in a circular orbit of radius \[R\] has a period of\[4\,\,h\]. Another satellite with orbital radius \[3R\] around the same planet will have a period (in hours)
A)
\[16\]
done
clear
B)
\[4\]
done
clear
C)
\[4\sqrt{27}\]
done
clear
D)
\[4\sqrt{8}\]
done
clear
View Answer play_arrow
question_answer 25) The freezer in a refrigerator is located at the top section so that
A)
the entire chamber of the refrigerator is cooled quickly due to convection
done
clear
B)
the motor is not heated
done
clear
C)
the heat gained from the environment is high
done
clear
D)
the heat gained from the environment is low
done
clear
View Answer play_arrow
question_answer 26) A monoatomic gas is suddenly compressed to \[{{(1/8)}^{th}}\] of its initial volume adiabatically. The ratio of its final pressure to the initial pressure is (Given the ratio of the specific heats of the given gas to be\[5/3)\]
A)
\[32\]
done
clear
B)
\[40/3\]
done
clear
C)
\[24/5\]
done
clear
D)
\[8\]
done
clear
View Answer play_arrow
question_answer 27) A Carnot engine takes heat from a reservoir at \[{{627}^{o}}C\] and rejects heat to a sink at\[{{27}^{o}}C\]. Its efficiency will be
A)
\[3/5\]
done
clear
B)
\[1/3\]
done
clear
C)
\[2/3\]
done
clear
D)
\[200/209\]
done
clear
View Answer play_arrow
question_answer 28) A \[30\,\,V,\,\,90\,\,W\] lamp is to be operated on a \[120\,\,V\]\[DC\] line. For proper glow, a resistor, of\[...\Omega \] should be connected in series with the lamp.
A)
\[40\]
done
clear
B)
\[10\]
done
clear
C)
\[20\]
done
clear
D)
\[30\]
done
clear
View Answer play_arrow
question_answer 29) A tuning fork \[A\] produces \[4\] beats/s with another tuning fork \[B\] of frequency\[320\,\,Hz\]. On filing one of the prongs of \[A,\,\,4\] beats/s are again heard when sounded with the same fork\[B\]. Then, the frequency of the fork \[A\] before filing is
A)
\[328\,\,Hz\]
done
clear
B)
\[316\,\,Hz\]
done
clear
C)
\[324\,\,Hz\]
done
clear
D)
\[320\,\,Hz\]
done
clear
View Answer play_arrow
question_answer 30) The sprinkling of water reduces slightly the temperature of a closed room because
A)
temperature of water is less than that of the room
done
clear
B)
specific heat of water is high
done
clear
C)
water has large latent heat of vaporisation
done
clear
D)
water is a bad conductor of heat
done
clear
View Answer play_arrow
question_answer 31) The equation of a simple harmonic wave is given by\[y=5\sin \frac{\pi }{2}(100t-x)\], where \[x\] and \[y\] are in metre and time is in second. The period of the wave in second will be
A)
\[0.04\]
done
clear
B)
\[0.01\]
done
clear
C)
\[1\]
done
clear
D)
\[5\]
done
clear
View Answer play_arrow
question_answer 32) The loudness and pitch of a sound note depends on
A)
intensity and frequency
done
clear
B)
frequency and number of harmonics
done
clear
C)
intensity and velocity
done
clear
D)
frequency and velocity
done
clear
View Answer play_arrow
question_answer 33) For ordinary terrestrial experiments, the observer in an inertial frame in the following cases is
A)
a child revolving in a giant wheel
done
clear
B)
a driver in a sports car moving with a constant high speed of \[200\,\,km{{h}^{-1}}\] on a straight rod
done
clear
C)
the pilot of an aeroplane which is taking off
done
clear
D)
a cyclist negotiating a sharp curve
done
clear
View Answer play_arrow
question_answer 34) A rectangular vessel when full of water, takes \[10\,\,\min \] to be emptied through an orifice in its bottom. How much time will it take to be emptied when half filled with water?
A)
\[9\,\,\min \]
done
clear
B)
\[7\,\,\min \]
done
clear
C)
\[5\,\,\min \]
done
clear
D)
\[3\,\,\min \]
done
clear
View Answer play_arrow
question_answer 35) If there were no gravity, which of the following will not be there for a fluid?
A)
Viscosity
done
clear
B)
Surface tension
done
clear
C)
Pressure
done
clear
D)
Archimedes' upward thrust
done
clear
View Answer play_arrow
question_answer 36) In a \[LCR\] series circuit, the potential difference between the terminals of the inductance is\[60\,\,V\], between the terminals of the capacitor is\[30\,\,V\] and that across the resistance is\[40\,\,V\]. Then, supply voltage will be equal to
A)
\[50\,\,V\]
done
clear
B)
\[70\,\,V\]
done
clear
C)
\[130\,\,V\]
done
clear
D)
\[10\,\,V\]
done
clear
View Answer play_arrow
question_answer 37) When deuterium and helium are subjected to an accelerating field simultaneously then
A)
both acquire same energy
done
clear
B)
deuterium accelerates faster
done
clear
C)
helium accelerates faster
done
clear
D)
neither of them is accelerated
done
clear
View Answer play_arrow
question_answer 38) A solenoid \[1.5\,\,m\] long and \[0.4\,\,cm\] in diameter possesses \[10\] turns per \[cm\] length. A current of \[5\,\,A\] falls through it. The magnetic field at the axis inside the solenoid is
A)
\[2\pi \times {{10}^{-3}}T\]
done
clear
B)
\[2\pi \times {{10}^{-5}}T\]
done
clear
C)
\[4\pi \times {{10}^{-2}}T\]
done
clear
D)
\[4\pi \times {{10}^{-3}}T\]
done
clear
View Answer play_arrow
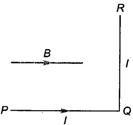
question_answer 39)
A wire \[PQR\] is bent as shown in figure and is placed in a region of uniform magnetic field\[B\]. The length of \[PQ=QR=l\,\,A\] current \[l\] ampere flows through the wire as shown. The magnitude of the force on \[PQ\] and \[QR\] will be
A)
\[BIl,\,\,0\]
done
clear
B)
\[BIl,\,\,0\]
done
clear
C)
\[0,\,\,BIl\]
done
clear
D)
\[0,\,\,0\]
done
clear
View Answer play_arrow
question_answer 40) A choke is preferred to a resistance for limiting current in \[AC\] circuit because
A)
choke is cheap
done
clear
B)
there is no wastage of power
done
clear
C)
choke is compact in size
done
clear
D)
choke is a good absorber of heat
done
clear
View Answer play_arrow
question_answer 41) If \[{{r}_{1}}\] and \[{{r}_{2}}\] are the radii of the atomic nuclei of mass numbers 64 and 125 respectively, then the ratio \[({{r}_{1}}/{{r}_{2}})\] is
A)
\[\frac{64}{125}\]
done
clear
B)
\[\sqrt{\frac{64}{125}}\]
done
clear
C)
\[\frac{5}{4}\]
done
clear
D)
\[\frac{4}{5}\]
done
clear
View Answer play_arrow
question_answer 42) A motor is used to deliver water at a certain rate through a given horizontal pipe. To deliver \[n-\]times the water through the same pipe in the same time the power of the motor must be increased as follows
A)
\[n-\]times
done
clear
B)
\[{{n}^{2}}-\]times
done
clear
C)
\[{{n}^{3}}-\]times
done
clear
D)
\[{{n}^{4}}-\]times
done
clear
View Answer play_arrow
question_answer 43) For a system to follow the law of conservation of linear momentum during a collision, the condition is (i) total external force acting on the system is zero. (ii) total external force acting on the system is finite and time of collision is negligible. (iii) total internal force acting on the system is zero.
A)
(i) only
done
clear
B)
(ii) only
done
clear
C)
(iii) only
done
clear
D)
(i) or (ii)
done
clear
View Answer play_arrow
question_answer 44) An air bubble of radius \[1\,\,cm\] rises from the bottom portion through a liquid of density \[1.5\,\,g/cc\] at a constant speed of\[0.25\,\,cm\,\,{{s}^{-1}}\]. If the density of air is neglected, the coefficient of viscosity of the liquid is approximately, (In\[Pa)\]
A)
\[13000\]
done
clear
B)
\[1300\]
done
clear
C)
\[130\]
done
clear
D)
\[13\]
done
clear
View Answer play_arrow
question_answer 45) A given mass of a gas is compressed isothermally until its pressure is doubled. It is then allowed to expand adiabatically until its original volume is restored and its pressure is then found to be \[0.75\] of its initial pressure. The ratio of the specific heats of the gas is approximately
A)
\[1.2\]
done
clear
B)
\[1.41\]
done
clear
C)
\[1.67\]
done
clear
D)
\[1.83\]
done
clear
View Answer play_arrow
question_answer 46) Two solid spheres \[A\] and \[B\] made of the same material have radii \[{{r}_{A}}\] and \[{{r}_{B}}\] respectively. Both the spheres are cooled from the same temperature under the conditions valid for Newton's law of cooling. The ratio of the rate of change of temperature \[A\] and \[B\] is
A)
\[\frac{{{r}_{A}}}{{{r}_{B}}}\]
done
clear
B)
\[\frac{{{r}_{B}}}{{{r}_{A}}}\]
done
clear
C)
\[\frac{r_{A}^{2}}{r_{B}^{2}}\]
done
clear
D)
\[\frac{r_{B}^{2}}{r_{A}^{2}}\]
done
clear
View Answer play_arrow
question_answer 47) The effect due to uniform magnetic field on a freely suspended magnetic needle is as follows
A)
both torque and net force are present
done
clear
B)
torque is present but no net force
done
clear
C)
both torque and net force are absent
done
clear
D)
net force is present but not torque
done
clear
View Answer play_arrow
question_answer 48) When a positively charged particle enters a uniform magnetic field with uniform velocity, its trajectory can be (i) a straight line (ii) a circle (iii) a helix
A)
(i) only
done
clear
B)
(i) or (ii)
done
clear
C)
(i) or (iii)
done
clear
D)
any one of (i), (ii) and (iii)
done
clear
View Answer play_arrow
question_answer 49) An oil drop having a mass \[4.8\times {{10}^{-10}}g\] and charge \[24\times {{10}^{-18}}C\] stands still between two charged horizontal plates separated by a distance of\[1\,\,cm\]. If now the polarity of the plates is changed, instantaneous acceleration of the drop is\[(g=10\,\,m{{s}^{-2}})\]
A)
\[5\,\,m{{s}^{-2}}\]
done
clear
B)
\[10\,\,m{{s}^{-2}}\]
done
clear
C)
\[15\,\,m{{s}^{-2}}\]
done
clear
D)
\[20\,\,m{{s}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 50) A free neutron decays spontaneously into
A)
a proton, an electron and anti-neutrino
done
clear
B)
a proton, an electron and a neutrino
done
clear
C)
a proton and electron
done
clear
D)
a proton, and electron, a neutrino and an anti-neutrino
done
clear
View Answer play_arrow
question_answer 51) What is the correct order of spin only magnetic moment (in\[BM)\]of\[M{{n}^{2+}},\,\,\,C{{r}^{2+}}\]and\[{{V}^{2+}}\]?
A)
\[M{{n}^{2+}}>{{V}^{2+}}>C{{r}^{2+}}\]
done
clear
B)
\[{{V}^{2+}}>C{{r}^{2+}}>M{{n}^{2+}}\]
done
clear
C)
\[M{{n}^{2+}}>C{{r}^{2+}}>{{V}^{2+}}\]
done
clear
D)
\[C{{r}^{2+}}>{{V}^{2+}}>M{{n}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 52) Which of the following is used for making optical instruments?
A)
\[Si{{O}_{2}}\]
done
clear
B)
\[Si\]
done
clear
C)
\[Si{{H}_{4}}\]
done
clear
D)
\[SiC\]
done
clear
View Answer play_arrow
question_answer 53) Which of the following is not correct?
A)
\[3{{O}_{2}}\underset{\text{discharge}}{\overset{\text{Silent}\,\,\text{electric}}{\longleftrightarrow}}2{{O}_{3}};\,\,\Delta H=-284.5\,\,kJ\]
done
clear
B)
Ozone undergoes addition reaction with unsaturated carbon compounds
done
clear
C)
Sodium thio sulphate reacts with \[{{I}_{2}}\] to form sodium tetrathionate and sodium iodide
done
clear
D)
Ozone oxidises lead sulphide to lead sulphate
done
clear
View Answer play_arrow
question_answer 54) Which of the following reactions can produce aniline as main product?
A)
\[{{C}_{6}}{{H}_{5}}N{{O}_{2}}+Zn/KOH\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}N{{O}_{2}}+Zn/N{{H}_{4}}Cl\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}N{{O}_{2}}+LiAl{{H}_{4}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}N{{O}_{2}}+Zn/HCl\]
done
clear
View Answer play_arrow
question_answer 55) Which of the following reagents when heated with ethyl chloride, forms ethylene?
A)
Aqueous\[KOH\]
done
clear
B)
\[Zn/HCl\]
done
clear
C)
Alcoholic\[KOH\]
done
clear
D)
\[HI\]
done
clear
View Answer play_arrow
question_answer 56) The energy of a photon is\[3\times {{10}^{-12}}erg\]. What is its wavelength in\[nm\]? \[(h=6.62\times {{10}^{-27}}erg-s;\,\,c=3\times {{10}^{10}}cm/s)\]
A)
\[66.2\]
done
clear
B)
\[1324\]
done
clear
C)
\[66.2\]
done
clear
D)
\[6.62\]
done
clear
View Answer play_arrow
question_answer 57) What is the time (in sec) required for depositing all the Silver present in \[125\,\,mL\] of \[1\,\,M\,\,AgN{{O}_{3}}\] solution by passing a current of\[241.25\,\,A\]? \[(1F=96500\,\,C)\]
A)
\[10\]
done
clear
B)
\[50\]
done
clear
C)
\[1000\]
done
clear
D)
\[100\]
done
clear
View Answer play_arrow
question_answer 58) The disperse phase, dispersion medium and nature of colloidal solution (lyophilic or lyophobic) of ?gold sol? respectively, are
A)
solid, solid, lyophobic
done
clear
B)
liquid, liquid, lyophobic
done
clear
C)
solid, liquid, lyophobic
done
clear
D)
solid, liquid, lyophilic
done
clear
View Answer play_arrow
question_answer 59) The rate constant of a first order reaction at \[{{27}^{o}}C\] is \[{{10}^{-3}}{{\min }^{-1}}\]. The temperature coefficient of this reaction is\[2\]. What is the rate constant (in\[{{\min }^{-1}})\] at \[{{17}^{o}}C\] for this reaction?
A)
\[{{10}^{-3}}\]
done
clear
B)
\[5\times {{10}^{-4}}\]
done
clear
C)
\[2\times {{10}^{-3}}\]
done
clear
D)
\[{{10}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 60) A solution of an acid has\[[{{H}^{+}}]=2\times {{10}^{-5}}\]. Find out the concentration of \[O{{H}^{-}}\] ions.
A)
\[5\times {{10}^{-10}}N\]
done
clear
B)
\[4\times {{10}^{-10}}N\]
done
clear
C)
\[2\times {{10}^{-5}}N\]
done
clear
D)
\[9\times {{10}^{-4}}N\]
done
clear
View Answer play_arrow
question_answer 61) Which of the following is added to chloroform to slow down its aerial oxidation in presence of light?
A)
Carbonyl chloride
done
clear
B)
Ethyl alcohol
done
clear
C)
Sodium hydroxide
done
clear
D)
Nitric acid
done
clear
View Answer play_arrow
question_answer 62) Which of the products is formed when acetone is reacted with barium hydroxide solution?
A)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,H-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,H-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}\]
done
clear
D)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} OH \\ | \end{smallmatrix}}{\mathop{C}}}\,-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} OH \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 63) When acetaldehyde is heated with Fehling solution, a red precipitate is formed. Which of the following is that?
A)
\[C{{u}_{2}}O\]
done
clear
B)
\[Cu\]
done
clear
C)
\[CuO\]
done
clear
D)
\[CuS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 64) What is the correct order of occurrence \[(%\] by weight) in air of\[Ne,\,\,\,Ar\]and\[Kr\]?
A)
\[Ne>Ar>Kr\]
done
clear
B)
\[Ar>Ne>Kr\]
done
clear
C)
\[Ar>Kr>Ne\]
done
clear
D)
\[Ne>Kr>Ar\]
done
clear
View Answer play_arrow
question_answer 65) Which of the following compounds when heated with \[CO\] at \[{{150}^{o}}C\] and \[500\,\,atm\] pressure in presence of \[B{{F}_{3}}\] forms ethyl propionate?
A)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
B)
\[C{{H}_{3}}OC{{H}_{3}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}\]
done
clear
D)
\[C{{H}_{3}}O{{C}_{2}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 66) Identify the reaction for which\[\Delta H\ne \Delta E\].
A)
\[S(\text{rhombic})+{{O}_{2}}(g)\xrightarrow{{}}S{{O}_{2}}(g)\]
done
clear
B)
\[{{N}_{2}}(g)+{{O}_{2}}(g)\xrightarrow{{}}2NO(g)\]
done
clear
C)
\[{{H}_{2}}(g)+C{{l}_{2}}(g)\xrightarrow{{}}2HCl(g)\]
done
clear
D)
\[CO(g)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow{{}}C{{O}_{2}}(g)\]
done
clear
View Answer play_arrow
question_answer 67) Hydrolysis of \[N{{O}_{3}}\] gives \[N{{H}_{3}}\] and\[X\]. Which of the following is\[X\]?
A)
\[HClO\]
done
clear
B)
\[HCl{{O}_{3}}\]
done
clear
C)
\[HOCl\]
done
clear
D)
\[HCl{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 68) What are the metal ions present in carnallite?
A)
\[Mg,\,\,K\]
done
clear
B)
\[Al,\,\,Na\]
done
clear
C)
\[Na,\,\,Mg\]
done
clear
D)
\[Zn,\,\,Mg\]
done
clear
View Answer play_arrow
question_answer 69) Ethyl chloride reacts with sodium ethoxide to form a compound\[A\]. Which of the following reactions also yields\[A?\]
A)
\[{{C}_{2}}{{H}_{5}}Cl,\,\,KOH(alc.),\,\,\Delta \]
done
clear
B)
\[2{{C}_{2}}{{H}_{5}}OH,\,\,conc.{{H}_{2}}S{{O}_{4}},\,\,{{140}^{o}}C\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}Cl,\,\,Mg\](dry ether)
done
clear
D)
\[{{C}_{2}}{{H}_{2}}dil.\,\,{{H}_{2}}S{{O}_{4}},\,\,HgS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 70) The number of sigma and \[pi(\pi )\] bonds present in benzene respectively are
A)
\[12,\,\,6\]
done
clear
B)
\[6,\,\,6\]
done
clear
C)
\[6,\,\,12\]
done
clear
D)
\[12,\,\,3\]
done
clear
View Answer play_arrow
question_answer 71) Edge length of a cube is \[400\,\,pm\], its body diagonal would be
A)
\[566\,\,pm\]
done
clear
B)
\[600\,\,pm\]
done
clear
C)
\[500\,\,pm\]
done
clear
D)
\[693\,\,pm\]
done
clear
View Answer play_arrow
question_answer 72) The number of \[\alpha -\]particles emitted by\[_{84}R{{a}^{218}}{{\xrightarrow{{}}}_{82}}P{{b}^{206}}\]
A)
\[3\]
done
clear
B)
\[4\]
done
clear
C)
\[6\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 73) The \[IUPAC\] name of the following compound is\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ {{C}_{6}}{{H}_{5}} \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{2}}-C{{H}_{3}}\]
A)
\[2-\]cyclohexylbutane
done
clear
B)
\[\sec -\]butylbenzene
done
clear
C)
\[3-\]cyclohexylbutane
done
clear
D)
\[2-\]phenylbutane
done
clear
View Answer play_arrow
question_answer 74) The reaction of primary amine with chloroform and ethanolic solution of \[KOH\] is called
A)
Hermann's reaction
done
clear
B)
Reimer-Tiemann's reaction
done
clear
C)
Carbylamine reaction
done
clear
D)
Kolbe's reaction
done
clear
View Answer play_arrow
question_answer 75) \[0.01\]mole of a non-electrolyte is dissolved in \[10\,\,g\] of water. The molality of the solution is
A)
\[0.1\,\,m\]
done
clear
B)
\[0.5\,\,m\]
done
clear
C)
\[1.0\,\,m\]
done
clear
D)
\[0.18\,\,m\]
done
clear
View Answer play_arrow
question_answer 76) Atoms with same atomic number and different mass numbers are called
A)
isobars
done
clear
B)
isomers
done
clear
C)
isotones
done
clear
D)
isotopes
done
clear
View Answer play_arrow
question_answer 77) The shape of the orbital with the value of\[l=2\] and \[m=0\]is
A)
spherical
done
clear
B)
dumb-bell
done
clear
C)
trigonal planar
done
clear
D)
square-planar
done
clear
View Answer play_arrow
question_answer 78) In the following, the element with the highest ionisation energy is
A)
\[[Ne]3{{s}^{2}}3{{p}^{1}}\]
done
clear
B)
\[[Ne]3{{s}^{2}}3{{p}^{3}}\]
done
clear
C)
\[[Ne]3{{s}^{2}}3{{p}^{2}}\]
done
clear
D)
\[[Ne]3{{s}^{2}}3{{p}^{4}}\]
done
clear
View Answer play_arrow
question_answer 79) In the conversion of \[B{{r}_{2}}\] to\[BrO_{3}^{-}\], the oxidation number of \[Br\] changes from
A)
zero to\[+5\]
done
clear
B)
\[+1\]to\[+5\]
done
clear
C)
zero to\[-3\]
done
clear
D)
\[+2\]to\[+5\]
done
clear
View Answer play_arrow
question_answer 80) Among the alkali metals cesium is the most reactive because
A)
its incomplete shell is nearest to the nucleus
done
clear
B)
it has a single electron in-the valence shell
done
clear
C)
it is the heaviest alkali metal
done
clear
D)
the outermost electron is more loosely bound than the outermost electron of the other alkali metals
done
clear
View Answer play_arrow
question_answer 81) Which of the following represents the Lewis structure of \[{{N}_{3}}\] molecule?
A)
\[_{\text{X}}^{\text{X}}N\equiv N_{\text{X}}^{\text{X}}\]
done
clear
B)
\[_{\text{X}}^{\text{X}}\overset{\text{X}\,\,\text{X}}{\mathop{N}}\,\equiv \overset{\text{X}\,\,\text{X}}{\mathop{N}}\,_{\text{X}}^{\text{X}}\]
done
clear
C)
\[_{\text{X}}^{\text{X}}\overset{\text{X}\,\,\text{X}}{\mathop{N}}\,_{\text{X}}^{\text{X}}-\underset{\text{X}\,\,\text{X}}{\mathop{\overset{\text{X}\,\,\text{X}}{\mathop{N}}\,_{\text{X}}^{\text{X}}}}\,\]
done
clear
D)
\[\underset{\text{X}\,\,\text{X}}{\mathop{_{\text{X}}^{\text{X}}\overset{\text{X}\,\,\text{X}}{\mathop{N}}\,}}\,_{\text{X}}^{\text{X}}-\underset{\text{X}\,\,\text{X}}{\mathop{\overset{\text{X}\,\,\text{X}}{\mathop{N}}\,_{\text{X}}^{\text{X}}}}\,\]
done
clear
View Answer play_arrow
question_answer 82) Hydrogen bond is strongest in
A)
\[S-H\cdot \cdot \cdot O\]
done
clear
B)
\[O-H\cdot \cdot \cdot S\]
done
clear
C)
\[F-H\cdot \cdot \cdot F\]
done
clear
D)
\[O-H\cdot \cdot \cdot N\]
done
clear
View Answer play_arrow
question_answer 83) The density of a gas is \[1.964\,\,d{{m}^{-3}}\] at \[273\,\,K\]and\[76\,\,cm\]\[Hg\]. The gas is
A)
\[C{{H}_{4}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
\[Xe\]
done
clear
View Answer play_arrow
question_answer 84) The shape of \[PC{{l}_{3}}\] molecule is
A)
trigonal bipyramidal
done
clear
B)
tetrahedral
done
clear
C)
pyramidal
done
clear
D)
square planar
done
clear
View Answer play_arrow
question_answer 85) The concentration of a reactant \[X\] decreases from \[0.1\,\,M\] to \[0.005\,\,M\] in\[40\,\,\min \]. If the reaction follows first order kinetics, the rate of the reaction when the concentration of \[X\] is \[0.01\,\,M\] will be
A)
\[1.73\times {{10}^{-4}}M\,\,{{\min }^{-1}}\]
done
clear
B)
\[3.47\times {{10}^{-4}}M\,\,{{\min }^{-1}}\]
done
clear
C)
\[3.47\times {{10}^{-5}}M\,\,{{\min }^{-1}}\]
done
clear
D)
\[7.5\times {{10}^{-4}}M\,\,{{\min }^{-1}}\]
done
clear
View Answer play_arrow
question_answer 86) Which of the following does not conduct electricity?
A)
Fused\[NaCl\]
done
clear
B)
Solid\[NaCl\]
done
clear
C)
Brine solution
done
clear
D)
Copper
done
clear
View Answer play_arrow
question_answer 87) Solubility product of a salt \[AB\] is \[1\times {{10}^{-8}}{{M}^{2}}\] in a solution in which the concentration of \[{{A}^{+}}\] ions is\[{{10}^{-3}}M\]. The salt will precipitate when the concentration of \[{{B}^{-}}\] ions is kept
A)
between\[{{10}^{-8}}M\]to\[{{10}^{-7}}M\]
done
clear
B)
between\[{{10}^{-7}}M\]to\[{{10}^{-8}}M\]
done
clear
C)
\[>{{10}^{-5}}M\]
done
clear
D)
\[<{{10}^{-8}}M\]
done
clear
View Answer play_arrow
question_answer 88) The \[pH\] of \[{{10}^{-8}}M\,\,HCl\] Solution is
A)
\[8\]
done
clear
B)
more than\[8\]
done
clear
C)
between \[6\] and \[7\]
done
clear
D)
slightly more than\[7\]
done
clear
View Answer play_arrow
question_answer 89) For a reaction to be spontaneous, at all temperatures
A)
\[\Delta G\] and \[\Delta H\] should be negative
done
clear
B)
\[\Delta G\] and \[\Delta H\] should be positive
done
clear
C)
\[\Delta G=\Delta S=0\]
done
clear
D)
\[\Delta H<\Delta G\]
done
clear
View Answer play_arrow
question_answer 90) Which of the following electrolytes will have maximum flocculation value for\[Fe{{(OH)}_{3}}\]sol?
A)
\[NaCl\]
done
clear
B)
\[N{{a}_{2}}S\]
done
clear
C)
\[{{(N{{H}_{4}})}_{3}}P{{O}_{4}}\]
done
clear
D)
\[{{K}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 91) What is the order of a reaction which has a rate expression\[rate=k{{[A]}^{3/2}}{{[B]}^{-1}}\]?
A)
\[\frac{3}{2}\]
done
clear
B)
\[\frac{1}{2}\]
done
clear
C)
\[0\]
done
clear
D)
\[\frac{4}{2}\]
done
clear
View Answer play_arrow
question_answer 92) Inductive effect involves
A)
displacement of \[\sigma -\]electrons
done
clear
B)
delocalisation of \[\pi -\]electrons
done
clear
C)
delocalisation of \[\sigma -\]electrons
done
clear
D)
displacement of \[\pi -\]electrons
done
clear
View Answer play_arrow
question_answer 93) Which of the following compound is expected to be optically active?
A)
\[{{(C{{H}_{3}})}_{2}}CHCHO\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CHO\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}CHBrCHO\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}CB{{r}_{2}}CHO\]
done
clear
View Answer play_arrow
question_answer 94) The catalyst used in the preparation of an alkyl chloride by the action of dry HC1 on an alcohol is
A)
anhydrous\[AlC{{l}_{3}}\]
done
clear
B)
\[FeC{{l}_{3}}\]
done
clear
C)
anhydrous\[ZnC{{l}_{2}}\]
done
clear
D)
\[Cu\]
done
clear
View Answer play_arrow
question_answer 95) By heating phenol with chloroform in alkali, it is converted into
A)
salicylic acid
done
clear
B)
salicylaldehyde
done
clear
C)
anisole
done
clear
D)
phenyl benzoate
done
clear
View Answer play_arrow
question_answer 96) Which of the following does not give benzoic acid on hydrolysis?
A)
Phenyl cyanide
done
clear
B)
Benzoyl chloride
done
clear
C)
Benzyl chloride
done
clear
D)
Methyl benzoate
done
clear
View Answer play_arrow
question_answer 97) Glucose contains in addition to aldehyde group
A)
one secondary \[-OH\] and four primary\[-OH\] groups
done
clear
B)
one primary \[-OH\] and four secondary \[-OH\] groups
done
clear
C)
two primary \[-OH\] and three secondary\[-OH\] groups
done
clear
D)
three primary \[-OH\] and two secondary \[-OH\] groups
done
clear
View Answer play_arrow
question_answer 98) The formula mass of Mohr's salt is\[392\]. The iron present in it is oxidised by \[KMn{{O}_{4}}\] in acid medium. The equivalent mass of Mohr's salt is
A)
\[392\]
done
clear
B)
\[31.6\]
done
clear
C)
\[278\]
done
clear
D)
\[156\]
done
clear
View Answer play_arrow
question_answer 99) The brown ring test for nitrates depends on
A)
the reduction of nitrate to nitric oxide
done
clear
B)
oxidation of nitric oxide to nitrogen dioxide
done
clear
C)
reduction of ferrous sulphate to iron
done
clear
D)
oxidising action of sulphuric acid
done
clear
View Answer play_arrow
question_answer 100) Which of the following solutions will exhibit highest boiling point?
A)
\[0.01\,\,M\,\,N{{a}_{2}}S{{O}_{4}}(aq)\]
done
clear
B)
\[0.01\,\,M\,\,KN{{O}_{3}}(aq)\]
done
clear
C)
\[0.015\,\,M\,\,urea(aq)\]
done
clear
D)
\[0.015\,\,M\,\,glucose(aq)\]
done
clear
View Answer play_arrow
question_answer 101) \[\int_{0}^{\pi /2}{\frac{x\sin x\cdot \cos x}{{{\cos }^{4}}x+{{\sin }^{4}}x}}dx\]is equal to
A)
\[\frac{{{\pi }^{2}}}{8}\]
done
clear
B)
\[\frac{{{\pi }^{2}}}{16}\]
done
clear
C)
\[1\]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow
question_answer 102) Equation of the tangent to the hyperbola\[2{{x}^{2}}-3{{y}^{2}}=6\]. Which is parallel to the line \[y-3x-4=0\]is
A)
\[y=3x+8\]
done
clear
B)
\[y=3x-8\]
done
clear
C)
\[y=3x+2\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 103) If the coefficient of correlation between two variables is\[0.32\], covariance is \[8\] and variance of \[x\] is\[25\], then variance of \[y\] is
A)
\[36\]
done
clear
B)
\[25\]
done
clear
C)
\[64\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 104) Ten coins are thrown simultaneously, the probability of getting at least \[7\] heads is
A)
\[\frac{63}{256}\]
done
clear
B)
\[\frac{121}{172}\]
done
clear
C)
\[\frac{113}{512}\]
done
clear
D)
\[\frac{11}{64}\]
done
clear
View Answer play_arrow
question_answer 105) When \[{{b}_{yx}}=0.03\] and\[{{b}_{xy}}=0.3\], then \[r\] is equal to approximately
A)
\[0.003\]
done
clear
B)
\[0.095\]
done
clear
C)
\[0.3\]
done
clear
D)
\[-0.3\]
done
clear
View Answer play_arrow
question_answer 106)
Use Simpson's\[\frac{1}{3}\]rule to find the value of \[\int_{1}^{5}{f(x)}\,\,dx\]given \[x\] \[1\] \[2\] \[3\] \[4\] \[4\] \[y\] \[10\] \[50\] \[70\] \[80\] \[100\]
A)
\[140.88\]
done
clear
B)
\[256.66\]
done
clear
C)
\[160.26\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 107) The feasible region represented by\[{{x}_{1}}+{{x}_{2}}\le 1\],\[-3{{x}_{1}}+{{x}_{2}}\ge 3,\,\,({{x}_{1}},\,\,{{x}_{2}}\ge 0)\]is
A)
a polygon
done
clear
B)
a singleton set
done
clear
C)
empty set
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 108) The points on the curve \[{{x}^{2}}=2y\] which are closest to the point \[(0,\,\,5)\] are
A)
\[(2,\,\,2),\,\,(-2,\,\,2)\]
done
clear
B)
\[(2\sqrt{2},\,\,4),\,\,(-2\sqrt{2},\,\,4)\]
done
clear
C)
\[(\sqrt{6},\,\,3),\,\,(-\sqrt{6},\,\,3)\]
done
clear
D)
\[(2\sqrt{3},\,\,6),\,\,(-2\sqrt{3},\,\,6)\]
done
clear
View Answer play_arrow
question_answer 109) Solve\[\left( \frac{dy}{dx} \right)\tan y=\sin (x+y)+\sin (x-y)\]
A)
\[\sec x-\frac{1}{2}\tan y=c\]
done
clear
B)
\[\log \sin (x+y)=c\]
done
clear
C)
\[\sec x+\tan y=c\]
done
clear
D)
\[\sec y+2\cos x=c\]
done
clear
View Answer play_arrow
question_answer 110) \[\overset{\to }{\mathop{\mathbf{A}}}\,\cdot \{(\overset{\to }{\mathop{\mathbf{B}}}\,+\overset{\to }{\mathop{\mathbf{C}}}\,)\times (\overset{\to }{\mathop{\mathbf{A}}}\,+\overset{\to }{\mathop{\mathbf{B}}}\,+\overset{\to }{\mathop{\mathbf{C}}}\,)\}\]equals
A)
\[[\overset{\to }{\mathop{\mathbf{A}}}\,\overset{\to }{\mathop{\mathbf{B}}}\,\overset{\to }{\mathop{\mathbf{C}}}\,]\]
done
clear
B)
\[[\overset{\to }{\mathop{\mathbf{B}}}\,\overset{\to }{\mathop{\mathbf{A}}}\,\overset{\to }{\mathop{\mathbf{C}}}\,]\]
done
clear
C)
\[0\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 111) If\[y=\frac{{{y}^{3}}}{3}+\frac{{{y}^{5}}}{5}+...\infty =2\left[ x+\frac{{{x}^{3}}}{3}+\frac{{{x}^{5}}}{5}+...\infty \right]\] then value of \[y\] is
A)
\[\frac{x}{1-{{x}^{2}}}\]
done
clear
B)
\[\frac{2x}{1+{{x}^{2}}}\]
done
clear
C)
\[\frac{1-{{x}^{2}}}{2x}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 112) If\[x=\frac{1}{2}(\sqrt{3}+i)\], then \[{{x}^{3}}\] is equal to
A)
\[1\]
done
clear
B)
\[-1\]
done
clear
C)
\[i\]
done
clear
D)
\[-i\]
done
clear
View Answer play_arrow
question_answer 113) if the complex numbers \[\sin x+i\cos 2x\] and \[\cos x-i\sin 2x\] are complex conjugate to each other, then the value of \[x\] is
A)
\[\frac{\pi }{4}\]
done
clear
B)
\[\frac{\pi }{8}\]
done
clear
C)
\[\frac{3\pi }{4}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 114) \[\underset{x\to {{2}^{+}}}{\mathop{\lim }}\,\frac{|x-2|}{x-2}\]is equal to
A)
\[-1\]
done
clear
B)
\[1\]
done
clear
C)
\[2\]
done
clear
D)
\[-2\]
done
clear
View Answer play_arrow
question_answer 115) If\[\left| \begin{matrix} x & {{x}^{2}} & 1+{{x}^{3}} \\ y & {{y}^{2}} & 1+{{y}^{3}} \\ z & {{z}^{2}} & 1+{{z}^{3}} \\ \end{matrix} \right|=0\], then
A)
\[z=xy\]
done
clear
B)
\[z=\frac{1}{xy}\]
done
clear
C)
\[z=-\frac{1}{xy}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 116) \[{{\cos }^{4}}\frac{\pi }{8}+{{\cos }^{4}}\frac{3\pi }{8}+{{\cos }^{4}}\frac{5\pi }{8}+{{\cos }^{4}}\frac{7\pi }{8}\]is equal to
A)
\[\frac{3}{2}\]
done
clear
B)
\[-\frac{2}{3}\]
done
clear
C)
\[-1\]
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 117) In a triangle\[ABC\], if\[\tan \frac{A}{2}=\frac{5}{6}\]and\[\tan \frac{B}{2}=\frac{20}{37}\], then \[a+c\]is equal to
A)
\[b\]
done
clear
B)
\[2b\]
done
clear
C)
\[3b\]
done
clear
D)
\[4b\]
done
clear
View Answer play_arrow
question_answer 118) Number of solutions of the equation \[\tan x+\sec x=2\cos x\] lying in the interval\[[0,\,\,2\pi ]\]is
A)
\[0\]
done
clear
B)
\[1\]
done
clear
C)
\[2\]
done
clear
D)
\[3\]
done
clear
View Answer play_arrow
question_answer 119) Equation of the pair of straight lines bisecting the angles between the lines represented by \[a{{x}^{2}}+hxy+b{{y}^{2}}=0\]is
A)
\[\frac{{{x}^{2}}-{{y}^{2}}}{a-b}=\frac{2xy}{h}\]
done
clear
B)
\[\frac{{{x}^{2}}+{{y}^{2}}}{a+b}=\frac{xy}{2h}\]
done
clear
C)
\[\frac{{{x}^{2}}-{{y}^{2}}}{a-b}=\frac{xy}{h}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 120) The equation of circle which touches the axes and the line \[\frac{x}{3}+\frac{y}{4}=1\] and whose centre lies in the first quadrant is\[{{x}^{2}}+{{y}^{2}}-2cx-2cy+{{c}^{2}}=0\]. Then, \[c\] is equal to
A)
\[1\]
done
clear
B)
\[2\]
done
clear
C)
\[3\]
done
clear
D)
.\[6\]
done
clear
View Answer play_arrow
question_answer 121) Angle between any two diagonals of a cube is
A)
\[\frac{\pi }{3}\]
done
clear
B)
\[{{\cos }^{-1}}\left( \frac{1}{3} \right)\]
done
clear
C)
\[{{\cos }^{-1}}\left( \frac{1}{\sqrt{3}} \right)\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 122) If`\[{{a}_{1}},\,\,{{a}_{2}},\,\,{{a}_{3}},...,{{a}_{n}}\]are in\[AP\]. Where\[{{a}_{i}}>0\]for all\[i\]. Find the sum of series \[\frac{1}{\sqrt{{{a}_{1}}}+\sqrt{{{a}_{2}}}}+\frac{1}{\sqrt{{{a}_{2}}}+\sqrt{{{a}_{3}}}}+\frac{1}{\sqrt{{{a}_{3}}+{{a}_{4}}}}+...\]\[+\frac{1}{\sqrt{{{a}_{n-1}}}+\sqrt{{{a}_{n}}}}\]
A)
\[\frac{n+1}{\sqrt{{{a}_{1}}+{{a}_{n}}}}\]
done
clear
B)
\[\frac{n-1}{\sqrt{{{a}_{1}}}-\sqrt{{{a}_{n}}}}\]
done
clear
C)
\[\frac{n+1}{\sqrt{{{a}_{1}}}+\sqrt{{{a}_{n}}}}\]
done
clear
D)
\[\frac{n-1}{\sqrt{{{a}_{1}}}+\sqrt{{{a}_{n}}}}\]
done
clear
View Answer play_arrow
question_answer 123) The ratio in which the \[xy-\]plane meets the line joining the points \[(-3,\,\,4,\,\,-8)\] and \[(5,\,\,-6,\,\,4)\]is
A)
\[2:3\]
done
clear
B)
\[2:1\]
done
clear
C)
\[4:5\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 124) The unit vector perpendicular to each of the vectors\[3\widehat{\mathbf{i}}+\widehat{\mathbf{j}}+2\widehat{\mathbf{k}}\], and\[2\widehat{\mathbf{i}}-2\widehat{\mathbf{j}}+4\widehat{\mathbf{k}}\]
A)
\[\frac{\widehat{\mathbf{i}}-\widehat{\mathbf{j}}-\widehat{\mathbf{k}}}{\sqrt{3}}\]
done
clear
B)
\[\frac{\widehat{\mathbf{i}}+\widehat{\mathbf{j}}+\widehat{\mathbf{k}}}{\sqrt{3}}\]
done
clear
C)
\[\frac{\widehat{\mathbf{i}}+\widehat{\mathbf{j}}-\widehat{\mathbf{k}}}{\sqrt{3}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 125) \[y={{\tan }^{-1}}\frac{\sqrt{(1+{{x}^{2}})}+\sqrt{(1-{{x}^{2}})}}{\sqrt{(1+{{x}^{2}})}-\sqrt{(1-{{x}^{2}})}}\], then\[\frac{dy}{dx}\]is equal to
A)
\[\frac{1}{\sqrt{1+{{x}^{2}}}}\]
done
clear
B)
\[-\frac{1}{2}\]
done
clear
C)
\[-\frac{x}{\sqrt{(1-{{x}^{4}})}}\]
done
clear
D)
\[\frac{x\sqrt{1+{{x}^{2}}}}{1-{{x}^{4}}}\]
done
clear
View Answer play_arrow
question_answer 126) \[\overrightarrow{\mathbf{a}},\,\,\overrightarrow{\mathbf{b}},\,\,\overrightarrow{\mathbf{c}}\] are coplanar vectors, then which of the following is not correct?
A)
\[\overrightarrow{\mathbf{a}}\cdot (\overrightarrow{\mathbf{b}}\times \overrightarrow{\mathbf{c}})=0\]
done
clear
B)
\[\overrightarrow{\mathbf{a}}\times (\overrightarrow{\mathbf{b}}\times \overrightarrow{\mathbf{c}})=0\]
done
clear
C)
\[[\overrightarrow{\mathbf{a}}+\overrightarrow{\mathbf{b}},\,\,\overrightarrow{\mathbf{b}}+\overrightarrow{\mathbf{c}},\,\,\overrightarrow{\mathbf{c}}+\overrightarrow{\mathbf{a}}]=0\]
done
clear
D)
\[\overrightarrow{\mathbf{a}}=p\overrightarrow{\mathbf{b}}+q\overrightarrow{\mathbf{c}}\]
done
clear
View Answer play_arrow
question_answer 127) If\[|A|\ne 0\]and \[A\] is of order n, then\[adj\,\,(adj\,\,A)\]is equal to
A)
\[|A{{|}^{n}}\]
done
clear
B)
\[|A{{|}^{2}}\]
done
clear
C)
\[|A{{|}^{n-1}}I\]
done
clear
D)
\[|A{{|}^{n-2}}\cdot A\]
done
clear
View Answer play_arrow
question_answer 128) \[\int{\frac{x+\sin x}{1+\cos x}}dx\]is equal to
A)
\[x\log (1+\cos x)+c\]
done
clear
B)
\[\frac{1}{x}\log (1+\cos x)+c\]
done
clear
C)
\[x\tan \frac{x}{2}+c\]
done
clear
D)
\[{{x}^{2}}{{\tan }^{-1}}\frac{x}{2}+c\]
done
clear
View Answer play_arrow
question_answer 129) Find the differential equation of curves \[y=A{{e}^{x}}+B{{e}^{-x}}\] for different values of \[A\] and \[B\]
A)
\[\frac{{{d}^{2}}y}{d{{x}^{2}}}-2y=0\]
done
clear
B)
\[\frac{{{d}^{2}}y}{d{{x}^{2}}}=y\]
done
clear
C)
\[\frac{{{d}^{2}}y}{d{{x}^{2}}}=4y+3\]
done
clear
D)
\[\frac{{{d}^{2}}y}{d{{x}^{2}}}+y=0\]
done
clear
View Answer play_arrow
question_answer 130) Solve\[\frac{dy}{dx}=\frac{{{y}^{2}}}{xy-{{x}^{2}}}\]
A)
\[y=c{{e}^{x/y}}\]
done
clear
B)
\[y=c{{e}^{-y/x}}+x\]
done
clear
C)
\[y=c{{e}^{y/x}}\]
done
clear
D)
\[xy=c{{e}^{y/x}}\]
done
clear
View Answer play_arrow
question_answer 131) If\[\underset{x\to a}{\mathop{\lim }}\,\frac{{{a}^{x}}-{{x}^{a}}}{{{x}^{x}}-{{a}^{a}}}=-1\], then
A)
\[a=1\]
done
clear
B)
\[a=0\]
done
clear
C)
\[a=e\]
done
clear
D)
\[a=\frac{1}{e}\]
done
clear
View Answer play_arrow
question_answer 132) If four digits are taken from the digits \[1,\,\,2,\,\,3,\,\,4,\] \[5,\,\,6,\,\,7\]. The probability that the sum of digits is less than\[12\], is
A)
\[\frac{3}{25}\]
done
clear
B)
\[\frac{4}{35}\]
done
clear
C)
\[\frac{2}{35}\]
done
clear
D)
\[\frac{1}{35}\]
done
clear
View Answer play_arrow
question_answer 133) The probability of happening exactly one of a two events \[A\] and \[B\] is
A)
\[P(A)+P(B)-2P(A\cap B)\]
done
clear
B)
\[P(A)+P(B)-P(A\cap B)\]
done
clear
C)
\[P(A)-P(B)\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 134) Solve\[x\cos x\left( \frac{dy}{dx} \right)+y(x\sin x+\cos x)=1\]
A)
\[y=x\tan x+\sin x+c\]
done
clear
B)
\[x=y\tan x+c\]
done
clear
C)
\[yx\sec x=\tan x+c\]
done
clear
D)
\[xy\cos x=x+c\]
done
clear
View Answer play_arrow
question_answer 135) If the equation\[({{a}^{2}}+4a+3){{x}^{2}}+({{a}^{2}}-a-2)x\]\[+a(a+1)=0\]has more than two roots, then value of \[a\] is
A)
\[0\]
done
clear
B)
\[1\]
done
clear
C)
\[-1\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 136) \[{{S}_{1}},\,\,{{S}_{2}}\]and \[{{S}_{3}}\] are the sums of\[n,\,\,2n\] and \[3n\]terms of an arithmetic progression respectively, then
A)
\[{{S}_{2}}=3{{S}_{3}}-2{{S}_{1}}\]
done
clear
B)
\[{{S}_{3}}=4({{S}_{1}}+{{S}_{2}})\]
done
clear
C)
\[{{S}_{3}}=3({{S}_{2}}-{{S}_{1}})\]
done
clear
D)
\[{{S}_{3}}=2({{S}_{2}}+{{S}_{1}})\]
done
clear
View Answer play_arrow
question_answer 137) If\[^{n}{{C}_{r-1}}=36,\,{{\,}^{n}}{{C}_{r}}=84\]and\[^{n}{{C}_{r+1}}=126\], then \[n\] is equal to
A)
\[8\]
done
clear
B)
\[9\]
done
clear
C)
\[10\]
done
clear
D)
\[11\]
done
clear
View Answer play_arrow
question_answer 138) The sides \[AB,\,\,BC,\,\,CA\] of triangle \[ABC\] have \[3,\,\,\,4\] and \[5\] interior points respectively on them. Find the number of triangles that can be constructed using these points as vertices
A)
\[201\]
done
clear
B)
\[120\]
done
clear
C)
\[205\]
done
clear
D)
\[435\]
done
clear
View Answer play_arrow
question_answer 139) Coefficient of \[{{x}^{n}}\] in the expansion of\[1+\frac{a+bx}{1!}+\frac{{{(a+bx)}^{2}}}{2!}+\frac{{{(a+bx)}^{3}}}{3!}+...\]is
A)
\[\frac{{{e}^{a}}{{b}^{n}}}{n!}\]
done
clear
B)
\[\frac{{{(b\cdot a)}^{n}}}{n}\]
done
clear
C)
\[\frac{{{e}^{b}}\cdot {{b}^{n}}}{(n-1)!}\]
done
clear
D)
\[\frac{{{a}^{n}}\cdot {{b}^{n-1}}}{n!}\]
done
clear
View Answer play_arrow
question_answer 140) \[\frac{{{C}_{0}}}{1}+\frac{{{C}_{2}}}{3}+\frac{{{C}_{4}}}{5}+\frac{{{C}_{6}}}{7}+...\]is equal to
A)
\[\frac{{{2}^{n-1}}}{n-1}\]
done
clear
B)
\[\frac{{{2}^{n+1}}}{n+3}\]
done
clear
C)
\[\frac{{{2}^{n}}}{n+1}\]
done
clear
D)
\[\frac{{{2}^{n-2}}}{n}\]
done
clear
View Answer play_arrow
question_answer 141) The equation of the parabola having the focus at the point \[(3,\,\,-1)\] and the vertex at \[(2,\,\,-1)\] is
A)
\[{{y}^{2}}-4x-2y+9=0\]
done
clear
B)
\[{{y}^{2}}+4x+2y-9=0\]
done
clear
C)
\[{{y}^{2}}-4x+2y+9=0\]
done
clear
D)
\[{{y}^{2}}+4x-2y+9=0\]
done
clear
View Answer play_arrow
question_answer 142) The equation of lines joining the origin to the points of intersection of \[y=x+3\] and \[4{{x}^{2}}+4{{y}^{2}}=1\] is
A)
\[36({{x}^{2}}+{{y}^{2}})={{(x-y)}^{2}}\]
done
clear
B)
\[12({{x}^{2}}+{{y}^{2}})={{(x+y)}^{2}}\]
done
clear
C)
\[9({{x}^{2}}+{{y}^{2}})=4{{(x+y)}^{2}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 143) The angle of elevation of a jet fighter from a point \[A\] on the ground is\[{{60}^{o}}\]. After a flight of\[10\,\,s\], the angle of elevation changes to\[{{30}^{o}}\]. If the jet is flying at a speed of\[432\,\,km/h\]. Find the constant height at which the jet is flying.
A)
\[200\sqrt{3}m\]
done
clear
B)
\[400\sqrt{3}m\]
done
clear
C)
\[600\sqrt{3}m\]
done
clear
D)
\[800\sqrt{3}m\]
done
clear
View Answer play_arrow
question_answer 144) Which is incorrect?
A)
\[(AB)'=B'A'\]
done
clear
B)
\[{{(AB)}^{\theta }}={{B}^{\theta }}{{A}^{\theta }}\]
done
clear
C)
\[\overline{AB}=\bar{B}\bar{A}\]
done
clear
D)
\[{{(AB)}^{-1}}={{B}^{-1}}{{A}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 145) Find the equation of plane through the line\[\frac{x-2}{2}=\frac{y-3}{3}=\frac{z-4}{5}\]and parallel to \[x-\]axis.
A)
\[2x+3y+5z=1\]
done
clear
B)
\[2x-5y=4\]
done
clear
C)
\[5y-3z-3=0\]
done
clear
D)
\[3y+4z=0\]
done
clear
View Answer play_arrow
question_answer 146) Find the moment of the force\[5\widehat{\mathbf{i}}+10\widehat{\mathbf{j}}+16\widehat{\mathbf{k}}\] acting at the point\[2\widehat{\mathbf{i}}-7\widehat{\mathbf{j}}+10\widehat{\mathbf{k}}\], about the point \[-5\widehat{\mathbf{i}}+6\widehat{\mathbf{j}}-10\widehat{\mathbf{k}}\]
A)
\[41\widehat{\mathbf{i}}-8\widehat{\mathbf{j}}+55\widehat{\mathbf{k}}\]
done
clear
B)
\[-408\widehat{\mathbf{i}}-12\widehat{\mathbf{j}}+135\widehat{\mathbf{k}}\]
done
clear
C)
\[-36\widehat{\mathbf{i}}+14\widehat{\mathbf{j}}-35\widehat{\mathbf{k}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 147) Find the equation of tangents to the ellipse\[\frac{{{x}^{2}}}{{{a}^{2}}}+\frac{{{y}^{2}}}{{{b}^{2}}}=1\], which cut off equal intercepts or the axes.
A)
\[y=\sqrt{3}x\pm \sqrt{3{{a}^{2}}+{{b}^{2}}}\]
done
clear
B)
\[y=\pm x\mp \sqrt{{{a}^{2}}+{{b}^{2}}}\]
done
clear
C)
\[y=\sqrt{3}\pm \sqrt{{{a}^{2}}+3{{b}^{2}}}\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 148) A square matrix \[A\] is called an orthogonal matrix if
A)
\[A\bar{A}=I\]
done
clear
B)
\[AA'=I\]
done
clear
C)
\[A{{A}^{\theta }}=I\]
done
clear
D)
\[{{A}^{2}}=I\]
done
clear
View Answer play_arrow
question_answer 149) The value of \['c'\] Rolle's theorem for \[f(x)={{e}^{x}}\sin x\]in\[[0,\,\,\pi ]\]is given by
A)
\[\frac{\pi }{4}\]
done
clear
B)
\[\frac{3\pi }{4}\]
done
clear
C)
\[\frac{5\pi }{6}\]
done
clear
D)
\[\frac{\pi }{2}\]
done
clear
View Answer play_arrow
question_answer 150) \[\int_{-a}^{a}{x\sqrt{({{a}^{2}}-{{x}^{2}})}}dx\]is equal to
A)
\[\frac{\pi }{4}\]
done
clear
B)
\[\frac{\pi }{3}\]
done
clear
C)
\[\frac{\pi }{8}\]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow