question_answer 1) L, C and R represent physical quantities inductance, capacitance and resistance respectively. The combination representing dimension of frequency is
A)
LC
done
clear
B)
\[{{(LC)}^{-1/2}}\]
done
clear
C)
\[{{(L/C)}^{-1/2}}\]
done
clear
D)
\[\text{C/L}\]
done
clear
View Answer play_arrow
question_answer 2) Energy of a photon is given by \[\text{E=hv}\] where v is the number of vibrations per second and h is Planck's constant. Dimensions of Planck's constant are
A)
\[\text{ }\!\![\!\!\text{ }{{\text{M}}^{\text{0}}}{{\text{L}}^{\text{0}}}{{\text{T}}^{\text{0}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
B)
\[\text{ }\!\![\!\!\text{ M}{{\text{L}}^{2}}{{\text{T}}^{\text{-1}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
C)
\[\text{ }\!\![\!\!\text{ M}{{\text{L}}^{2}}{{\text{T}}^{\text{-2}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
D)
\[\text{ }\!\![\!\!\text{ M}{{\text{L}}^{2}}{{\text{T}}^{\text{-3}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
View Answer play_arrow
question_answer 3) A particle moving with a uniform acceleration travels 24 m and 64 m in the first two consecutive intervals of 4s each. Its initial velocity is
A)
1m/s
done
clear
B)
10m/s
done
clear
C)
5 m/s
done
clear
D)
2 m/s
done
clear
View Answer play_arrow
question_answer 4) A bullet is fired with a speed of 1000 m/s in order to hit a target 100 m away. If g = 10 m/s2. The gun should be aimed
A)
directly towards the target
done
clear
B)
5 cm above the target
done
clear
C)
10 cm above the target
done
clear
D)
15 cm above the target
done
clear
View Answer play_arrow
question_answer 5) A particle moves in the \[\text{xy-}\]plane under the action of a force \[\mathbf{\vec{F}}\] such that the components of its linear momentum \[\mathbf{\vec{P}}\] at any time t are \[{{P}_{x}}=2\,\cos \,t,\,{{P}_{y}}=2\,\sin \,t.\]The angle between F and p at time t is
A)
\[90{}^\circ \]
done
clear
B)
\[0{}^\circ \]
done
clear
C)
\[180{}^\circ \]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
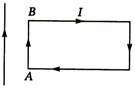
question_answer 6) A uniform rope of length L, resting on a frictionless horizontal surface, is pulled at one end by a force F. The tension in the rope at a distance I from this end is
A)
F
done
clear
B)
\[\frac{l}{L}F\]
done
clear
C)
\[\frac{L}{l}F\]
done
clear
D)
\[\left( 1-\frac{l}{L} \right)F\]
done
clear
View Answer play_arrow
question_answer 7) A bicycle is in motion. The frictional force exerted by the ground on the two wheels acts
A)
backwards on the front wheel and forwards on the rear wheel
done
clear
B)
forwards on the front wheel and backwards on the rear wheel
done
clear
C)
backwards on both the wheel
done
clear
D)
forwards on both the wheels
done
clear
View Answer play_arrow
question_answer 8) If the momentum of a body increases by 50%, its kinetic energy will increase by
A)
50%
done
clear
B)
100%
done
clear
C)
125%
done
clear
D)
150%
done
clear
View Answer play_arrow
question_answer 9) In which case does the potential energy decrease?
A)
On compressing a spring
done
clear
B)
On stretching a spring
done
clear
C)
On moving a body against gravitational force
done
clear
D)
On the rising of an air bubble in water
done
clear
View Answer play_arrow
question_answer 10) A body is moved from rest along a straight line by a machine delivering constant power. The distance moved by the body in time t is proportional to
A)
\[{{t}^{1/2}}\]
done
clear
B)
\[{{t}^{3/4}}\]
done
clear
C)
\[{{t}^{3/2}}\]
done
clear
D)
\[{{t}^{2}}\]
done
clear
View Answer play_arrow
question_answer 11) The root-mean-square velocity for an ideal gas is (where the symbols have their usual meanings)
A)
\[{{v}_{rms}}=\sqrt{\frac{3MT}{R}}\]
done
clear
B)
\[{{v}_{rms}}=\sqrt{\frac{3RT}{M}}\]
done
clear
C)
\[{{v}_{rms}}=\sqrt{\frac{3RM}{T}}\]
done
clear
D)
\[{{v}_{rms}}=\sqrt{3RMT}\]
done
clear
View Answer play_arrow
question_answer 12) The radius of a rotating disc is suddenly reduced to half without any change in its mass. Then its angular velocity will be
A)
four times
done
clear
B)
double
done
clear
C)
half
done
clear
D)
unchanged
done
clear
View Answer play_arrow
question_answer 13) A heavy mass is attached to a thin wire and is whirled in a vertical circle. The wire is most likely to break
A)
when the mass is at the highest point of the circle
done
clear
B)
when the mass is at the lowest point of the circle
done
clear
C)
when the wire is horizontal
done
clear
D)
at an angle of cos-1 (1/3) from the upward vertical
done
clear
View Answer play_arrow
question_answer 14) The ratio of the weight of a man in a stationary lift and when it is moving downward with uniform acceleration \[a\] is 3 : 2. The value of \[a\] is (g = acceleration due to gravity of the earth)
A)
3/2 g
done
clear
B)
g/3
done
clear
C)
2/3 g
done
clear
D)
g
done
clear
View Answer play_arrow
question_answer 15) The masses and radii of the earth and moon are \[{{M}_{1}},\,{{R}_{1}}\] and \[{{M}_{2}},\,{{R}_{2}}\] respectively. Their centres are distance d apart. The minimum velocity with which a particle of mass m should be projected from a point midway between their centres so that it escapes to infinity is
A)
\[\sqrt[2]{\frac{G}{a}({{M}_{1}}+{{M}_{2}})}\]
done
clear
B)
\[\sqrt[2]{\frac{2G}{a}({{M}_{1}}+{{M}_{2}})}\]
done
clear
C)
\[\sqrt[2]{\frac{Gm}{a}({{M}_{1}}+{{M}_{2}})}\]
done
clear
D)
\[\sqrt[2]{\frac{Gm({{M}_{1}}+{{M}_{2}})}{d({{R}_{1}}+{{R}_{2}})}}\]
done
clear
View Answer play_arrow
question_answer 16) A geostationary satellite is taken from its orbit to another orbit. If the distance of the second orbit from the centre of the earth is double that of the first orbit, then its time period in the second orbit will be
A)
4.8 h
done
clear
B)
48\[\sqrt{2}\] h
done
clear
C)
48 h
done
clear
D)
24 h
done
clear
View Answer play_arrow
question_answer 17) Two wires of the same material have lengths in the ratio 1 : 2 and their radii are in the ratio 1: \[\sqrt{2}\]. If they are streched by applying equal forces, the increase in their lengths will be in the ratio
A)
2:\[\sqrt{2}\]
done
clear
B)
\[\sqrt{2}\]:2
done
clear
C)
1:1
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
question_answer 18) The amount of work done in forming a soap film of size \[10cm\times 10cm\] (Surface tension \[T=3\times {{10}^{-2}}N/m\]) is
A)
\[6\times {{10}^{-4}}\text{J}\]
done
clear
B)
\[3\times {{10}^{-4}}\text{J}\]
done
clear
C)
\[6\times {{10}^{-3}}\text{J}\]
done
clear
D)
\[6\times {{10}^{-2}}\text{J}\]
done
clear
View Answer play_arrow
question_answer 19) A number of water droplets each of radius r coalesce to form a bigger drop of radius R. The rise in temperature is
A)
\[\frac{3T}{RJ}\]
done
clear
B)
\[\frac{3T}{rJ}\]
done
clear
C)
\[\frac{3T}{J}\left( \frac{1}{r}+\frac{1}{R} \right)\]
done
clear
D)
\[\frac{3T}{J}\left( \frac{1}{r}-\frac{1}{R} \right)\]
done
clear
View Answer play_arrow
question_answer 20) Air is filled at \[60{}^\circ C\] in a vessel of open mouth. The vessel is heated to a temperature T so that \[\frac{1}{4}\]th part of air escapes. The value of T is
A)
\[80{}^\circ C\]
done
clear
B)
\[444{}^\circ C\]
done
clear
C)
\[333{}^\circ C\]
done
clear
D)
\[171{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 21) The specific heat of a gas
A)
has only two values \[{{C}_{P}}\] and \[{{C}_{V}}\]
done
clear
B)
has a unique value at a given temperature
done
clear
C)
can have any value between 0 and \[\infty \]
done
clear
D)
depends upon the mass of the gas
done
clear
View Answer play_arrow
question_answer 22) The pressure of a gas is equal to
A)
the total translation kinetic energy of all the molecules in unit volume
done
clear
B)
the total kinetic energy of all the molecules in unit volume
done
clear
C)
two thirds of the total translational kinetic energy of all the molecules in unit volume
done
clear
D)
two-thirds of the total kinetic energy of all the molecules in unit volume
done
clear
View Answer play_arrow
question_answer 23) During the adiabatic expansion of 2 moles of a gas, the internal energy was found to have decreased by 100 J. The work done by the gas in this process is
A)
zero
done
clear
B)
- 100 J
done
clear
C)
200 J
done
clear
D)
100 J
done
clear
View Answer play_arrow
question_answer 24) Two samples A and B of a gas, initially at the same pressure and temperature, are compressed from volume V to V/2 (A isothermally and B adiabatically) the final pressure of A is
A)
greater than the final pressure of B
done
clear
B)
equal to the final pressure of B
done
clear
C)
less than the final pressure of B
done
clear
D)
twice the final pressure of B
done
clear
View Answer play_arrow
question_answer 25) When the potential energy of a particle executing simple harmonic motion is one-fourth of its maximum value during the oscillation, its displacement from the equilibrium position in terms of its amplitude \[a\] is
A)
\[a\text{/4}\]
done
clear
B)
\[a\text{/3}\]
done
clear
C)
\[a\text{/2}\]
done
clear
D)
\[2a\text{/3}\]
done
clear
View Answer play_arrow
question_answer 26) A wall has two layers A and B, each made of a different material. Both the layers have the same thickness. The thermal conductivity of the material of A is twice that of B. Under thermal equilibrium, the temperature difference across the wall is \[36{}^\circ C\]. The temperature difference across the layer A is
A)
\[6{}^\circ C\]
done
clear
B)
\[12{}^\circ C\]
done
clear
C)
\[18{}^\circ C\]
done
clear
D)
\[24{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 27) The temperature of two bodies A and B are respectively \[727{}^\circ C\] and \[327{}^\circ C\]. The ratio of rates of heat radiated by them is
A)
727:327
done
clear
B)
5 : 3
done
clear
C)
25 : 9
done
clear
D)
625 : 81
done
clear
View Answer play_arrow
question_answer 28) A body takes T minutes to cool from \[62{}^\circ C\] to \[61{}^\circ C\] when the surrounding temperature is \[30{}^\circ C\]. The time taken by the body to cool from \[46{}^\circ C\] to \[45.5{}^\circ C\] is
A)
greater than T minutes
done
clear
B)
equal to T minutes
done
clear
C)
less than T minutes
done
clear
D)
equal to T/2 minutes
done
clear
View Answer play_arrow
question_answer 29) 41forks are so arranged that each produces 5 beats/s when sounded with its near fork. If the frequency of last fork is double the frequency of first fork, then the frequencies of the first and last fork are respectively
A)
200, 400
done
clear
B)
205,410
done
clear
C)
195, 390
done
clear
D)
100, 200
done
clear
View Answer play_arrow
question_answer 30) A motor car blowing a horn of frequency 124 vib/s moves with a velocity 72 km/h towards a tall wall. The frequency of the reflected sound heard by the driver will be [velocity of sound in air is 330 m/s]
A)
109 vib/s
done
clear
B)
132 vib/s
done
clear
C)
140 vib/s
done
clear
D)
248 vib/s
done
clear
View Answer play_arrow
question_answer 31) Young's experiment establishes the fact that
A)
light consists of particles
done
clear
B)
light consists of waves
done
clear
C)
light consists of neither particles nor waves
done
clear
D)
fringe width do not depend on the separation of the two slits
done
clear
View Answer play_arrow
question_answer 32) In the context of Doppler effect in light, the term red shift signifies
A)
decrease in frequency
done
clear
B)
increase in frequency
done
clear
C)
decrease in intensity
done
clear
D)
increase in intensity
done
clear
View Answer play_arrow
question_answer 33) In a concave mirror experiment, an object is placed at a distance\[{{x}_{1}}\] from the focus and the image is formed at a distance \[{{x}_{2}}\] from the focus. The focal length of the mirror would be
A)
\[{{x}_{1}}{{x}_{2}}\]
done
clear
B)
\[\sqrt{{{x}_{1}}{{x}_{2}}}\]
done
clear
C)
\[\sqrt{{{x}_{1}}/{{x}_{2}}}\]
done
clear
D)
\[\frac{{{x}_{1}}+{{x}_{2}}}{2}\]
done
clear
View Answer play_arrow
question_answer 34) Which of the following is a correct relation?
A)
\[_{\text{a}}{{\text{ }\!\!\mu\!\!\text{ }}_{\text{r}}}{{\text{=}}_{\text{a}}}{{\text{ }\!\!\mu\!\!\text{ }}_{\text{w}}}\,\text{ }\!\!\times\!\!\text{ }{{\,}_{\text{r}}}{{\text{ }\!\!\mu\!\!\text{ }}_{\text{w}}}\]
done
clear
B)
\[_{\text{a}}{{\text{ }\!\!\mu\!\!\text{ }}_{\text{r}}}{{\times }_{\text{a}}}{{\mu }_{w}}\,{{=}_{\text{w}}}{{\text{ }\!\!\mu\!\!\text{ }}_{\text{a}}}\]
done
clear
C)
\[_{a}{{\text{ }\!\!\mu\!\!\text{ }}_{\text{r}}}/{{ }_{r}}{{\mu }_{a}}\,=0\]
done
clear
D)
\[_{\text{a}}{{\text{ }\!\!\mu\!\!\text{ }}_{\text{r}}}/{{ }_{w}}{{\mu }_{r}}\,{{=}_{a}}{{\text{ }\!\!\mu\!\!\text{ }}_{w}}\]
done
clear
View Answer play_arrow
question_answer 35) The focal length of a thin lens is \[f\] and the diameter of its aperture is d. This makes an image of brightness \[I\]. If the central portion of the aperture be covered upto d/2 diameter by an opaque paper, then the focal length and the brightness of image would respectively be
A)
\[f\text{/2}\,\text{and}\,\text{I/2}\]
done
clear
B)
\[f\,\text{and}\,\text{I/4}\]
done
clear
C)
\[f\text{/2}\,\text{and}\,\text{I/4}\]
done
clear
D)
\[f\,\text{and}\,3\text{I/4}\]
done
clear
View Answer play_arrow
question_answer 36) Fraunhofer lines are an example of
A)
continuous spectrum
done
clear
B)
band spectrum
done
clear
C)
emission spectrum
done
clear
D)
absorption spectrum
done
clear
View Answer play_arrow
question_answer 37) The reason for shining of air bubble in water is
A)
diffraction of light
done
clear
B)
dispersion of light
done
clear
C)
scattering of light
done
clear
D)
total internal reflection of light
done
clear
View Answer play_arrow
question_answer 38) The dispersive powers of glasses of lenses used in an achromatic pair are in the ratio 5:3. If the focal length of the concave lens is 15 cm, then the nature and focal length the other lens would be
A)
convex, 9 cm
done
clear
B)
convex, 9 cm
done
clear
C)
convex, 25 cm
done
clear
D)
convex, 25 cm
done
clear
View Answer play_arrow
question_answer 39) White light is passed through a prism whose angle is \[5{}^\circ \]. If the refractive indices for rays of red and blue colors are respectively 1.64 and 1.66, the angle of deviation between the two colors will be
A)
0.1 degree
done
clear
B)
0.2 degree
done
clear
C)
0.3 degree
done
clear
D)
0.4 degree
done
clear
View Answer play_arrow
question_answer 40) The resolving limit of healthy eye is about
A)
\[1'\]
done
clear
B)
\[1''\]
done
clear
C)
\[1{}^\circ \]
done
clear
D)
\[\frac{1}{60}''\]
done
clear
View Answer play_arrow
question_answer 41) While viewing a distant object with a telescope suddenly a housefly sits on objective lens. The correct statement is that
A)
housefly will be seen enlarged in image
done
clear
B)
housefly will be seen reduced in image
done
clear
C)
intensity of image will be decreased
done
clear
D)
intensity of image will be increased
done
clear
View Answer play_arrow
question_answer 42) An object of height 1.5 cm is placed on the axis of a convex lens of focal length 25 cm. A real image is formed at a distance of 75 cm from the lens. The size of the image will be
A)
4.5cm
done
clear
B)
3.0cm
done
clear
C)
0.75cm
done
clear
D)
0.5cm
done
clear
View Answer play_arrow
question_answer 43) Work function of a metal is 2.51 eV. Its threshold frequency is
A)
\[5.9\times {{10}^{14}}cycle/s\]
done
clear
B)
\[6.5\times {{10}^{14}}cycle/s\]
done
clear
C)
\[9.4\times {{10}^{14}}cycle/s\]
done
clear
D)
\[6.08\times {{10}^{14}}cycle/s\]
done
clear
View Answer play_arrow
question_answer 44) Energy conversion in a photoelectric cell takes place from
A)
chemical to electrical
done
clear
B)
magnetic to electrical
done
clear
C)
optical to electrical
done
clear
D)
mechanical to electrical
done
clear
View Answer play_arrow
question_answer 45) The momentum of a photon in an X-ray beam of \[{{10}^{-10}}m\] wavelength is
A)
\[1.5\times {{10}^{-23}}kg-m/s\]
done
clear
B)
\[6.6\times {{10}^{-24}}kg-m/s\]
done
clear
C)
\[6.6\times {{10}^{-44}}kg-m/s\]
done
clear
D)
\[2.2\times {{10}^{-52}}kg-m/s\]
done
clear
View Answer play_arrow
question_answer 46) The rest energy of an electron is 0.511 MeV The electron is accelerated from rest to a velocity 0.5 c. The change in its energy will be
A)
0.026 MeV
done
clear
B)
0.051 MeV
done
clear
C)
0.079 MeV
done
clear
D)
0.105 MeV
done
clear
View Answer play_arrow
question_answer 47) X-rays from X-ray tube
A)
will be monochromatic
done
clear
B)
will have all possible wavelengths less than a certain longest wavelength
done
clear
C)
will have all possible wavelengths greater than a certain shortest wavelength
done
clear
D)
will have all wavelengths between the longest and the shortest wavelength
done
clear
View Answer play_arrow
question_answer 48) X-ray beam of intensity \[{{I}_{0}}\] passes through absorption plate of thickness d. If absorption coefficient of material of plate is µ, the correct statement regarding the transmitted intensity \[I\] of X-ray is
A)
\[I={{I}_{0}}(1-{{e}^{-\mu d}})\]
done
clear
B)
\[I={{I}_{0}}{{e}^{-\mu d}}\]
done
clear
C)
\[I={{I}_{0}}e(1-{{e}^{-\mu /d}})\]
done
clear
D)
\[I={{I}_{0}}{{e}^{-\mu /d}}\]
done
clear
View Answer play_arrow
question_answer 49) At zero kelvin a piece of germanium
A)
becomes semiconductor
done
clear
B)
becomes good conductor
done
clear
C)
becomes bad conductor
done
clear
D)
has maximum conductivity
done
clear
View Answer play_arrow
question_answer 50) Electronic configuration of germanium is 2, 8, 18 and 4. To make it extrinsic semiconductor small quantity of antimony is added
A)
the material obtained will be n-type germanium in which electrons and holes are equal in number
done
clear
B)
the material obtained will be p-type germanium
done
clear
C)
the material obtained will be n-type germanium which has more electrons then holes at room temperature
done
clear
D)
the material obtained will be n-type germanium which has less electrons than holes at room temperature
done
clear
View Answer play_arrow
question_answer 51) A semiconductor is cooled from \[T{{ }_{1}}\]K to \[T{{ }_{2}}\]K its resistance
A)
will decrease
done
clear
B)
will increase
done
clear
C)
will first decrease and then increase
done
clear
D)
will not change
done
clear
View Answer play_arrow
question_answer 52) In a hydrogen atom an electron moves in a circular orbit of radius \[5.2\times {{10}^{-11}}m\] and produces a magnetic induction of 12.56 T at its nucleus. The current produced by the motion of the electron will be (Given \[{{\mu }_{0}}=4\pi \times {{10}^{-7}}\] Wb/ Am)
A)
\[6.53\times {{10}^{-}}\text{A}\]
done
clear
B)
\[13.25\times {{10}^{-10}}\text{A}\]
done
clear
C)
\[9.6\times {{10}^{6}}\text{A}\]
done
clear
D)
\[1.04\times {{10}^{-3}}\text{A}\]
done
clear
View Answer play_arrow
question_answer 53) The \[{{K}_{\alpha }}\] line for molybdenum (atomic no. = 42) has a wavelength of \[0.7078\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]. The wavelength of\[{{K}_{\alpha }}\] line of zinc (atomic no. = 30) will be
A)
\[1\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[1.388\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[0.3541\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[0.5\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 54) In a hydrogen atom, which of the following electronic transitions would involve the maximum energy change?
A)
from n = 2 to n = 1
done
clear
B)
from n = 3 to n = 1
done
clear
C)
from n = 4 to n = 2
done
clear
D)
from n = 3 to n = 2
done
clear
View Answer play_arrow
question_answer 55) In the lowest energy level of hydrogen atom, the electron has the angular momentum
A)
\[\text{ }\!\!\pi\!\!\text{ /h}\]
done
clear
B)
\[\text{h/ }\!\!\pi\!\!\text{ }\]
done
clear
C)
\[\text{h/2 }\!\!\pi\!\!\text{ }\]
done
clear
D)
\[\text{2 }\!\!\pi\!\!\text{ /h}\]
done
clear
View Answer play_arrow
question_answer 56) In a radioactive decay, neither the atomic number nor the mass number changes. Which of the following would be emitted in the decay process?
A)
Proton
done
clear
B)
Neutron
done
clear
C)
Electron
done
clear
D)
Photon
done
clear
View Answer play_arrow
question_answer 57) The half-life of a radio isotope is 5 yr. What fraction of this material would decay in 15 yr?
A)
1
done
clear
B)
¾
done
clear
C)
7/8
done
clear
D)
1/8
done
clear
View Answer play_arrow
question_answer 58) The disintegration constant of a radioactive element is\[\lambda .\]Its half-life and means life are respectively
A)
\[1\text{/ }\!\!\lambda\!\!\text{ }\] and In \[\text{2/ }\!\!\lambda\!\!\text{ }\]
done
clear
B)
In \[\text{2/ }\!\!\lambda\!\!\text{ }\] and \[\text{1/ }\!\!\lambda\!\!\text{ }\]
done
clear
C)
\[\text{ }\!\!\lambda\!\!\text{ }\] (In 2)and \[\text{1/ }\!\!\lambda\!\!\text{ }\]
done
clear
D)
\[\text{ }\!\!\lambda\!\!\text{ /}\](In 2) and \[\text{1/ }\!\!\lambda\!\!\text{ }\]
done
clear
View Answer play_arrow
question_answer 59) Magnetic lines of force
A)
always intersect
done
clear
B)
are always closed
done
clear
C)
tend to crowd far away from the poles of a magnet
done
clear
D)
do not pass through vacuum
done
clear
View Answer play_arrow
question_answer 60) Rate of change of torque t with deflection \[\text{ }\!\!\theta\!\!\text{ }\] is maximum for a magnet suspended freely in a uniform magnetic field of induction B, when
A)
\[\text{ }\!\!\theta\!\!\text{ }\,\text{=}\,{{\text{0}}^{\text{o}}}\]
done
clear
B)
\[\text{ }\!\!\theta\!\!\text{ }\,\text{=}\,{{45}^{0}}\]
done
clear
C)
\[\text{ }\!\!\theta\!\!\text{ }\,\text{=}\,{{60}^{0}}\]
done
clear
D)
\[\text{ }\!\!\theta\!\!\text{ }\,\text{=}\,{{90}^{0}}\]
done
clear
View Answer play_arrow
question_answer 61) The angle of dip at a place on the earth gives
A)
the horizontal component of the earth's magnetic field
done
clear
B)
the location of the geographic meridian
done
clear
C)
the vertical component of the earth's field
done
clear
D)
the direction of the earth's magnetic field
done
clear
View Answer play_arrow
question_answer 62) The tangent galvanometer, when connected in series with a standard resistance can be used as
A)
an ammeter
done
clear
B)
a voltmeter
done
clear
C)
a wattmeter
done
clear
D)
Both an ammeter and a voltmeter
done
clear
View Answer play_arrow
question_answer 63) In the vibration magnetometer, the magnet kept in the stirrup when disturbed vibrates with a period, given by
A)
\[2\pi \sqrt{I/MB}\]
done
clear
B)
\[2\pi \sqrt{MB/I}\]
done
clear
C)
\[2\pi \sqrt{MBI}\]
done
clear
D)
\[2\pi \sqrt{I/g}\]
done
clear
View Answer play_arrow
question_answer 64) When the symbols have their usual meaning. An electric dipole is put in north-south direction in a sphere filled with water. Which statement is correct?
A)
Electric flux is coming towards sphere
done
clear
B)
Electric flux is coming out of sphere
done
clear
C)
Electric flux entering into sphere and leaving the sphere are same
done
clear
D)
Water does not permit electric flux to enter into sphere
done
clear
View Answer play_arrow
question_answer 65) Deuteron and \[\alpha \]-particle are put 1 A apart in air, magnitude of intensity of electric field due to deuteron at \[\alpha \]-particle is
A)
\[2.88\times {{10}^{11}}\text{N/C}\]
done
clear
B)
\[1.44\times {{10}^{11}}\text{N/C}\]
done
clear
C)
\[5.76\times {{10}^{11}}\text{N/C}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 66) The permittivity and permeability of vacuum are \[{{\varepsilon }_{0}}\] and \[{{\mu }_{0}}\] respectively. The corresponding quantities for a medium are \[\varepsilon \] and\[\mu .\] Refractive index of a medium is
A)
\[\sqrt{\frac{\mu \varepsilon }{\mu {{ }_{0}}{{\varepsilon }_{0}}}}\]
done
clear
B)
\[\frac{\mu {{ }_{0}}{{\varepsilon }_{0}}}{\sqrt{\mu \varepsilon }}\]
done
clear
C)
\[\frac{\sqrt{\mu {{ }_{0}}{{\varepsilon }_{0}}}}{\mu \varepsilon }\]
done
clear
D)
\[\sqrt{\frac{\mu {{ }_{0}}{{\varepsilon }_{0}}}{\mu \varepsilon }}\]
done
clear
View Answer play_arrow
question_answer 67) A force F acts between sodium and chlorine ions of salt (sodium chloride) when put 1 cm apart in the air. The permittivity of air and dielectric constant of water are \[{{\varepsilon }_{0}}\] and K respectively. When a piece of salt is put in water electrical force acting between sodium and chlorine ion 1 cm apart is
A)
\[F\]
done
clear
B)
\[FK\text{/}{{\text{ }\!\!\varepsilon\!\!\text{ }}_{\text{0}}}\]
done
clear
C)
\[\text{F/K}{{\text{ }\!\!\varepsilon\!\!\text{ }}_{\text{0}}}\]
done
clear
D)
\[\text{F/K}\]
done
clear
View Answer play_arrow
question_answer 68) The radii of two spheres forming a spherical condenser are 0.5 and 0.6 m. If a medium having dielectric constant 6 is completely filled in between, the capacity of the condenser will be
A)
\[3.3\times {{10}^{-10}}\text{F}\]
done
clear
B)
\[2\times {{10}^{-9}}\text{F}\]
done
clear
C)
\[2\text{F}\]
done
clear
D)
\[\text{18F}\]
done
clear
View Answer play_arrow
question_answer 69) A parallel plate capacitor is filled up by a substance of\[{{t}_{1}}\] thickness of dielectric constant \[{{K}_{1}}\] and other substance of \[{{t}_{2}}\] thickness of dielectric constant \[{{K}_{2.}}\] The capacity of this capacitor will be
A)
\[{{\varepsilon }_{0}}A/\left( \frac{{{t}_{1}}}{{{K}_{1}}}+\frac{{{t}_{2}}}{{{K}_{2}}} \right)\]
done
clear
B)
\[{{\varepsilon }_{0}}A/\left( \frac{{{K}_{1}}}{{{K}_{2}}}+\frac{{{K}_{2}}}{{{t}_{2}}} \right)\]
done
clear
C)
\[{{\varepsilon }_{0}}A/\left( \frac{{{t}_{1}}}{{{K}_{2}}}+\frac{{{t}_{2}}}{{{K}_{1}}} \right)\]
done
clear
D)
\[{{\varepsilon }_{0}}A/\left( \frac{{{K}_{2}}}{{{t}_{1}}}+\frac{{{K}_{1}}}{{{t}_{2}}} \right)\]
done
clear
View Answer play_arrow
question_answer 70) n identical condensers are joined in parallel and are charged to potential V. Now, they are separated and joined in series. Then, the total energy and potential difference of the combination will be
A)
energy remain the same and potential difference is also same
done
clear
B)
energy remain the same and potential difference is \[nV\]
done
clear
C)
energy increases n times and potential difference remains is \[nV\]
done
clear
D)
energy increases n times and potential difference remains same
done
clear
View Answer play_arrow
question_answer 71) The electromotive force of primary cell is 2 V. When it is short circuited it gives a current of 4 A. Its internal resistance in ohm is
A)
0.5
done
clear
B)
5.0
done
clear
C)
2.0
done
clear
D)
8.0
done
clear
View Answer play_arrow
question_answer 72) A potential differences of V is applied at the ends of a copper wire of length \[l\]and diameter d. On doubling only d, drift velocity
A)
becomes two times
done
clear
B)
becomes half
done
clear
C)
does not change
done
clear
D)
becomes one-fourth
done
clear
View Answer play_arrow
question_answer 73)
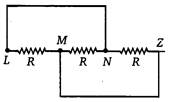
Three equal resistances each of value R. are joined as shown in the figure. The equivalent resistance between M and N is
A)
R
done
clear
B)
2R
done
clear
C)
R/2
done
clear
D)
R/3
done
clear
View Answer play_arrow
question_answer 74) In the measurement of a resistance by Wheatstone bridge the known and the unknown resistance are interchanged to neutralize
A)
end error
done
clear
B)
index error
done
clear
C)
error due to thermoelectric effect
done
clear
D)
random error
done
clear
View Answer play_arrow
question_answer 75) A thermoelectric couple is made from copper and iron. At hot junction, current
A)
flows from copper towards iron
done
clear
B)
flows from iron towards copper
done
clear
C)
flow decreases
done
clear
D)
flow increases
done
clear
View Answer play_arrow
question_answer 76) Which of the following is not a correct statement?
A)
Resistivity of electrolytes decreases on increasing temperature
done
clear
B)
Resistance of mercury falls on decreasing its temperature
done
clear
C)
When joined in series a 40 W bulb glows more than a 60W bulb
done
clear
D)
Resistance of 40 W bulb is less than the resistance of 60 W bulb
done
clear
View Answer play_arrow
question_answer 77) The relation between Farady constant F. electron charge e and Avogadro Number N is
A)
\[F=\text{N/e}\]
done
clear
B)
\[F=\text{Ne}\]
done
clear
C)
\[N=F{{\text{e}}^{2}}\]
done
clear
D)
\[F={{N}^{2}}\text{e}\]
done
clear
View Answer play_arrow
question_answer 78)
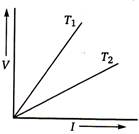
The voltage V and current \[I\] graph for s conductor at two different temperatures \[{{T}_{1}}\] and \[{{T}_{2}}\] are shown in the figure. The relation between \[{{T}_{1}}\] and \[{{T}_{2}}\] is
A)
\[{{T}_{1}}>{{T}_{2}}\]
done
clear
B)
\[{{T}_{1}}\approx {{T}_{2}}\]
done
clear
C)
\[{{T}_{1}}={{T}_{2}}\]
done
clear
D)
\[{{T}_{1}}<{{T}_{2}}\]
done
clear
View Answer play_arrow
question_answer 79) Two bulbs of 500 W and 200 W are manufactured to operate on 220 V line. The ratio of heat produced in 500 W and 200 W, in two cases, when firstly they are joined in parallel and secondly in series, will be
A)
5/2,2/5
done
clear
B)
5/2,5/2
done
clear
C)
2/5, 5/2
done
clear
D)
2/5, 2/5
done
clear
View Answer play_arrow
question_answer 80)
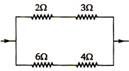
In the circuit as shown in the figure, the heat produced by \[6\,\,\Omega \] resistance due to current flowing in it is 60cal/s. The heat generated across\[3\,\,\Omega \] resistance per second will be
A)
30cal
done
clear
B)
60cal
done
clear
C)
100 cal
done
clear
D)
120 cal
done
clear
View Answer play_arrow
question_answer 81) The value of Faraday constant is
A)
\[9.65\times {{10}^{4}}C\]
done
clear
B)
\[9.65\times {{10}^{4}}\text{J}\]
done
clear
C)
\[6.03\times {{10}^{27}}\text{J}\]
done
clear
D)
\[1.6\times {{10}^{-19}}C\]
done
clear
View Answer play_arrow
question_answer 82) A proton and a deuteron both having the same kinetic energy, enter perpendicularly into a uniform magnetic field B. For motion of proton and deuteron on circular path of radius \[{{r}_{P}}\] and \[{{r}_{d}}\] respectively, the correct statement is
A)
\[{{r}_{d}}={{r}_{P}}\sqrt{2}\]
done
clear
B)
\[{{r}_{d}}\frac{{{r}_{P}}}{\sqrt{2}}\]
done
clear
C)
\[{{r}_{d}}={{r}_{P}}\]
done
clear
D)
\[{{r}_{d}}=2{{r}_{P}}\]
done
clear
View Answer play_arrow
question_answer 83) A short bar magnet placed with its axis at \[30{}^\circ \] with a uniform external magnetic field of 0.16 T experiences a torque of magnitude 0.032 J. The magnetic moment of the bar magnet will be
A)
0.23 J/T
done
clear
B)
0.40 J/T
done
clear
C)
0.80 J/T
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 84)
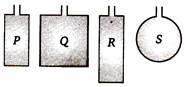
Four wires each of length 2.0 n are bent into four loops P, Q, R and S and then suspended into uniform magnetic field. Same current is passed in each loop. Which statement is correct?
A)
Couple on loop P will be the highest
done
clear
B)
Couple on loop Q will be the highest
done
clear
C)
Couple on loop R will be the highest
done
clear
D)
Couple on loop S will be the highest
done
clear
View Answer play_arrow
question_answer 85) Force acting between two point charges (+q and + q) distance r apart in the air is F. One charge is rotated about the other charge on a circle of radius r. The work done will be
A)
\[F\times r\]
done
clear
B)
\[F\times 2\pi r\]
done
clear
C)
\[F\times 2r\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 86)
A current \[I\] is flowing in a rectangular loop. It is put close to a wire (long and straight) such that wire is parallel to one of the arms of loop and lies in the plane of loop. If as shown in the figure, a steady current \[I\] flows in the wire, the loop
A)
will rotate about an axis parallel to wire
done
clear
B)
will move away from the wire
done
clear
C)
will move towards the wire
done
clear
D)
will remain steady
done
clear
View Answer play_arrow
question_answer 87) A current of 1 A is passed through a straight wire of length 2 m. The magnetic field at a point in air at a distance of 3 m from either end of wire and lying on the axis of wire will be
A)
\[\frac{{{\mu }_{0}}}{2\pi }\]
done
clear
B)
\[\frac{{{\mu }_{0}}}{4\pi }\]
done
clear
C)
\[\frac{{{\mu }_{0}}}{8\pi }\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 88) 5 cm long solenoid having \[10\,\Omega \] resistance and 5 mH inductance is joined to a 10 V battery. At steady state the current through the solenoid (in ampere) will be
A)
5
done
clear
B)
1
done
clear
C)
2
done
clear
D)
zero
done
clear
View Answer play_arrow
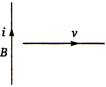
question_answer 89)
A conducting wire is moving towards right in a magnetic field B. The direction of induced current in the wire is shown in the figure. The direction of magnetic field will be
A)
in the plane of paper pointing towards right
done
clear
B)
in the plane of paper pointing towards left
done
clear
C)
perpendicular to the plane of paper and downwards
done
clear
D)
perpendicular to the plane of paper and upwards
done
clear
View Answer play_arrow
question_answer 90) The north pole of a bar magnet is moved swiftly downward towards a closed coil and then second time it is raised upwards slowly. The magnitude and direction of the induced currents in the two cases will be
A)
First case Second case Low value clockwise Higher value anticlockwise
done
clear
B)
First case Second case Low value clockwise Equal value anticlockwise
done
clear
C)
First case Second case Higher value clockwise low value anticlockwise
done
clear
D)
First case Second case Higher value anticlockwise Low value clockwise
done
clear
View Answer play_arrow
question_answer 91) "The direction of the inducedcurrentina circuit is always such that it opposes the cause due to which it is produced." This law is named as
A)
Ohm's Law
done
clear
B)
Lenz's Law
done
clear
C)
Kirchhoffs Law
done
clear
D)
Faraday's Law
done
clear
View Answer play_arrow
question_answer 92) At a place the value of horizontal component of the earth's magnetic field H is \[3\times {{10}^{-5}}Wb/{{m}^{2}}.\] A metallic rod AB of length 2m placed in east-west direction, having the end A towards east, falls vertically downward with a constant velocity of 50 m/s. Which end of the rod becomes positively charged and what is the value of induced potential difference between the two ends?
A)
\[EndA,3\times {{10}^{-3}}mV\]
done
clear
B)
End A, 3 mV
done
clear
C)
\[End\text{ }B,3\times {{10}^{-3}}mV\]
done
clear
D)
End B, 3 mV
done
clear
View Answer play_arrow
question_answer 93) A circular coil of radius 5 cm has 500 turns of a wire. The approximate value of the coefficient of self-induction of the coil will be
A)
25 mH
done
clear
B)
\[25\times {{10}^{-3}}mH\]
done
clear
C)
\[50\times {{10}^{-3}}mH\]
done
clear
D)
\[50\times {{10}^{-3}}mH\]
done
clear
View Answer play_arrow
question_answer 94) An emf of 100 mV is induced in a coil when the current in another nearby coil becomes 10 A from zero in 0.1 s. The coefficient of mutual induction between the two coils will be
A)
1mH
done
clear
B)
10 mH
done
clear
C)
100 mH
done
clear
D)
1000 mH
done
clear
View Answer play_arrow
question_answer 95) A light source approaches the observer with velocity 0.8 c. The Doppler shift for the light of wavelength \[5500\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\] is
A)
\[5500\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[1833\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[4400\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[7333\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 96) Two plates are 2 cm apart, a potential difference of 10 V is applied between them, the electric field between the plates is
A)
20N/C
done
clear
B)
500 N/C
done
clear
C)
5 N/C
done
clear
D)
250 N/C
done
clear
View Answer play_arrow
question_answer 97) A 120 V AC source is connected across a pure inductor of inductance 0.70 H. If the frequency of the source is 60 Hz, the current passing through the inductor is
A)
4.55 A
done
clear
B)
0.355 A
done
clear
C)
0.455 A
done
clear
D)
3.55 A
done
clear
View Answer play_arrow
question_answer 98) An AC generator produced an output voltage e = 170 sin 377 t volt, where t is in second. The frequency of AC voltage is
A)
50 Hz
done
clear
B)
110 Hz
done
clear
C)
60 Hz
done
clear
D)
230 Hz
done
clear
View Answer play_arrow
question_answer 99) In L-R circuit, for the case of increasing current, the magnitude of current can be calculated by using the formula
A)
\[I={{I}_{0}}{{e}^{-Rt/L}}\]
done
clear
B)
\[I={{I}_{0}}(1-{{e}^{-Rt/L}}\]
done
clear
C)
\[I={{I}_{0}}(1-{{e}^{Rt/L}})\]
done
clear
D)
\[I={{I}_{0}}{{e}^{Rt/L}}\]
done
clear
View Answer play_arrow
question_answer 100) The efficiency of transformer is very high because
A)
there is no moving part in a transformer
done
clear
B)
it produces very high voltage
done
clear
C)
it produces very low voltage
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 101) Which is correct statement about proton?
A)
Proton is nucleus of deuterium
done
clear
B)
Proton is alpha particle
done
clear
C)
proton is ionized hydrogen molecule
done
clear
D)
Proton is ionized hydrogen
done
clear
View Answer play_arrow
question_answer 102) In a given atom no two electrons can have the same values of all the quantum numbers. This is called
A)
Hund's rule
done
clear
B)
Aufbau principle
done
clear
C)
Uncertainty principle
done
clear
D)
Pauli's exclusion principle
done
clear
View Answer play_arrow
question_answer 103) Magnetic quantum number specifies
A)
size of orbitals
done
clear
B)
shape of orbitals
done
clear
C)
orientation of orbitals
done
clear
D)
nuclear stability
done
clear
View Answer play_arrow
question_answer 104) The energy of an electron in nth orbit of hydrogen atom is
A)
\[\frac{13.6}{{{n}^{4}}}eV\]
done
clear
B)
\[\frac{13.6}{{{n}^{3}}}eV\]
done
clear
C)
\[-\frac{13.6}{{{n}^{2}}}eV\]
done
clear
D)
\[\frac{13.6}{n}eV\]
done
clear
View Answer play_arrow
question_answer 105) If wavelength of photon is \[2.2\times {{10}^{-11}}m,\] \[h=6.6\times {{10}^{-34}}Js,\] then momentum of photon is
A)
\[3\times {{10}^{-23}}kg\,m{{s}^{-1}}\]
done
clear
B)
\[3.33\times {{10}^{22}}kg\,m{{s}^{-1}}\]
done
clear
C)
\[1.452\times {{10}^{-44}}\,kg\,m{{s}^{-1}}\]
done
clear
D)
\[6.89\times {{10}^{43}}kg\,m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 106) The bond order of \[NO\]molecule is
A)
\[1.5\]
done
clear
B)
\[2.0\]
done
clear
C)
\[2.5\]
done
clear
D)
\[3.0\]
done
clear
View Answer play_arrow
question_answer 107) The bond angle in water \[({{H}_{2}}O)\] molecule is
A)
\[{{109}^{o}}28'\]
done
clear
B)
\[{{180}^{o}}\]
done
clear
C)
\[{{90}^{o}}\]
done
clear
D)
\[{{105}^{o}}\]
done
clear
View Answer play_arrow
question_answer 108) The triple bond between N atoms of nitrogen molecule \[(N\equiv N)\] consists of
A)
three \[\sigma \]-bond
done
clear
B)
two \[\sigma \]-bonds and one \[\pi \]-bond
done
clear
C)
one \[\sigma \]-bond and two \[\pi \]-bonds
done
clear
D)
three \[\pi \]-bonds
done
clear
View Answer play_arrow
question_answer 109) According to VSEPR theory the most probable shape of the molecule having 4 electron pairs in the outer shell of the central atom is
A)
linear
done
clear
B)
tetrahedral
done
clear
C)
hexahedral
done
clear
D)
octahedral
done
clear
View Answer play_arrow
question_answer 110) The number of \[\sigma \] bonds in o-xylene is
A)
\[6\]
done
clear
B)
\[9\]
done
clear
C)
\[12\]
done
clear
D)
\[18\]
done
clear
View Answer play_arrow
question_answer 111) \[50\text{ }mL\]\[10\text{ }N\] \[{{H}_{2}}S{{O}_{4}},\]\[25\text{ }mL\]\[12N\] \[HCl\]and \[40\text{ }mL\]\[5N\]\[HN{{O}_{3}}\] were mixed together and the volume of the mixture was made \[1000\text{ }mL\]by adding water. The normality of the resultant solution will be
A)
\[1\text{ }N\]
done
clear
B)
\[\text{2 }N\]
done
clear
C)
\[\text{3 }N\]
done
clear
D)
\[\text{4 }N\]
done
clear
View Answer play_arrow
question_answer 112) Which of the following will have the highest boiling point at 1 atm pressure?
A)
\[0.1\,M\,\,NaCl\]
done
clear
B)
\[0.1\text{ }M\text{ }sucrose\]
done
clear
C)
\[0.1\text{ }M\text{ }BaC{{l}_{2}}\]
done
clear
D)
\[0.1\text{ }M\text{ }glucose\]
done
clear
View Answer play_arrow
question_answer 113) What will be the molality of a solution having \[18\text{ }g\]of glucose (mol. wt.\[=180\]) dissolved in \[500g\]of water?
A)
\[1m\]
done
clear
B)
\[0.5m\]
done
clear
C)
\[0.2m\]
done
clear
D)
\[2m\]
done
clear
View Answer play_arrow
question_answer 114) An aqueous solution freezes at \[-{{0.186}^{o}}C\] \[({{k}_{f}}={{1.86}^{o}};\,{{k}_{b}}={{0.512}^{o}})\]What is the elevation in boiling point?
A)
\[0.186\]
done
clear
B)
\[0.512\]
done
clear
C)
\[\frac{0.512}{1.86}\]
done
clear
D)
\[0.0512\]
done
clear
View Answer play_arrow
question_answer 115) Which one of the following is a colligative property?
A)
Surface tension
done
clear
B)
Osmotic pressure
done
clear
C)
Viscosity
done
clear
D)
Refractive index
done
clear
View Answer play_arrow
question_answer 116) How many kinds of space lattices are possible in a crystal?
A)
\[23\]
done
clear
B)
\[7\]
done
clear
C)
\[230\]
done
clear
D)
\[14\]
done
clear
View Answer play_arrow
question_answer 117) Diamond is an example of
A)
covalent solid
done
clear
B)
electrovalent solid
done
clear
C)
solid with hydrogen bonding
done
clear
D)
glass
done
clear
View Answer play_arrow
question_answer 118) What is Y in the following nuclear reaction? \[_{7}{{H}^{14}}{{+}_{1}}{{H}^{1}}{{\xrightarrow{{}}}_{8}}{{O}^{15}}+'X'\]
A)
\[_{0}{{n}^{1}}\]
done
clear
B)
\[_{-1}{{e}^{0}}\]
done
clear
C)
\[_{+1}{{e}^{0}}\]
done
clear
D)
Energy
done
clear
View Answer play_arrow
question_answer 119) The end product of \[(4n+2)\] radioactive disintegration series is
A)
\[_{82}^{208}Pb\]
done
clear
B)
\[_{82}^{206}Pb\]
done
clear
C)
\[_{82}^{207}Pb\]
done
clear
D)
\[_{83}^{210}Bi\]
done
clear
View Answer play_arrow
question_answer 120) The radioactive isotope which is used to estimate the age of ancient geological formations is
A)
\[{{O}^{8}}\]
done
clear
B)
\[P{{b}^{212}}\]
done
clear
C)
\[F{{e}^{59}}\]
done
clear
D)
\[_{6}{{C}^{14}}\]
done
clear
View Answer play_arrow
question_answer 121) The half-life period of a radioactive element is \[140\]days. In \[560\,days\]a sample of this element would be reduced to ......of its initial mass.
A)
\[1/2\]
done
clear
B)
\[1/4\]
done
clear
C)
\[1/8\]
done
clear
D)
\[1/16\]
done
clear
View Answer play_arrow
question_answer 122) For the homogeneous gas reaction, \[4N{{H}_{3}}+5{{O}_{2}}\rightleftharpoons 4NO+6{{H}_{2}}O\] the equilibrium constant \[{{K}_{c}},\] has the dimension of
A)
\[conc{{.}^{+10}}\]
done
clear
B)
\[conc{{.}^{+1}}\]
done
clear
C)
\[conc{{.}^{-1}}\]
done
clear
D)
it is dimensionless
done
clear
View Answer play_arrow
question_answer 123) Formation of the \[S{{O}_{3}}\] takes place according to the reaction \[2S{{O}_{2}}+{{O}_{2}}\rightleftharpoons 2S{{O}_{3}};\] \[\Delta H=-45.2kcal\] Which of the following factors favours the formation of \[S{{O}_{3}}\]?
A)
Increase in temperature
done
clear
B)
Increase in pressure
done
clear
C)
Removal of oxygen
done
clear
D)
Increase in volume
done
clear
View Answer play_arrow
question_answer 124) In the equilibrium reaction, \[2HI(g)\rightleftharpoons {{H}_{2}}+{{I}_{2}}\] which of the following expressions is true?
A)
\[{{K}_{p}}={{K}_{c}}\]
done
clear
B)
\[{{K}_{c}}=2{{K}_{p}}\]
done
clear
C)
\[{{K}_{p}}>{{K}_{c}}\]
done
clear
D)
\[{{K}_{c}}={{K}_{p}}{{(RT)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 125) \[{{K}_{p}}/{{K}_{c}}\]for the reaction,\[CO(g)+\frac{1}{2}{{O}_{2}}(g)\rightleftharpoons C{{O}_{2}}(g)\] is
A)
\[1\]
done
clear
B)
\[RT\]
done
clear
C)
\[\frac{1}{\sqrt{RT}}\]
done
clear
D)
\[{{(RT)}^{1/2}}\]
done
clear
View Answer play_arrow
question_answer 126) A monoprotic acid in a \[0.1\text{ }M\]solution ionises to \[0.001%\]. Its ionisation constant is
A)
\[1.0\times {{10}^{-3}}\]
done
clear
B)
\[1.0\times {{10}^{-6}}\]
done
clear
C)
\[1.0\times {{10}^{-8}}\]
done
clear
D)
\[1.0\times {{10}^{-11}}\]
done
clear
View Answer play_arrow
question_answer 127) The pH of a \[{{10}^{-8}}\] molar solution of \[HCl\]in water is
A)
\[8\]
done
clear
B)
\[-8\]
done
clear
C)
between \[7\] and \[8\]
done
clear
D)
between \[6\] and \[7\]
done
clear
View Answer play_arrow
question_answer 128) Which of the following salts when dissolved in water hydrolyse?
A)
\[NaCl\]
done
clear
B)
\[N{{H}_{4}}Cl\]
done
clear
C)
\[KCl\]
done
clear
D)
\[N{{a}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 129) What is the correct representation for the solubility product of \[Sn{{S}_{2}}\]?
A)
\[[S{{n}^{2+}}]\,{{[{{S}^{2-}}]}^{2}}\]
done
clear
B)
\[[S{{n}^{4+}}]\,{{[{{S}^{2-}}]}^{2}}\]
done
clear
C)
\[[S{{n}^{2+}}]\,[2{{S}^{2-}}]\]
done
clear
D)
\[[S{{n}^{2+}}]\,{{[2{{S}^{2-}}]}^{2}}\]
done
clear
View Answer play_arrow
question_answer 130) If \[100\text{ }mL\] of \[1N\] hydrochloric acid were mixed with \[100\text{ }mL\] of \[1M\]sodium hydroxide, the solution will be
A)
acidic
done
clear
B)
basic
done
clear
C)
neutral
done
clear
D)
slightly acidic
done
clear
View Answer play_arrow
question_answer 131) Which of the following expressions represents the first law of thermodynamics?
A)
\[q=\Delta E-W\]
done
clear
B)
\[\Delta E=q-W\]
done
clear
C)
\[\Delta E=q+W\]
done
clear
D)
\[\Delta E=q+pdV\]
done
clear
View Answer play_arrow
question_answer 132) In a spontaneous process the entropy of the system and its surroundings
A)
equals zero
done
clear
B)
decreases
done
clear
C)
increases
done
clear
D)
remains constant
done
clear
View Answer play_arrow
question_answer 133) For the first order reaction with the constant k, which expression gives the half-life period? (Initial concentration = a)
A)
\[\frac{\ln \,2}{k}\]
done
clear
B)
\[\frac{1}{k\times a}\]
done
clear
C)
\[\frac{0.693}{k\times a}\]
done
clear
D)
\[\frac{3}{2k{{a}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 134) The rate constant is given by the equation \[k=PZ{{e}^{-E/RT}},\] which factor should register a decrease for the reaction to proceed more rapidly?
A)
\[T\]
done
clear
B)
\[Z\]
done
clear
C)
\[E\]
done
clear
D)
\[P\]
done
clear
View Answer play_arrow
question_answer 135) For a reaction of the type \[A+B\to \]products, it is observed that doubling the concentration of A causes the reaction rate to be four times as great, but doubling the amount of B does not affect the rate. The rate equation is
A)
\[Rate=k[A]\,[B]\]
done
clear
B)
\[Rate=\frac{k}{4}{{[A]}^{2}}\]
done
clear
C)
\[Rate=k{{[A]}^{2}}[B]\]
done
clear
D)
\[Rate=k{{[A]}^{2}}{{[B]}^{2}}\]
done
clear
View Answer play_arrow
question_answer 136) Oxidation number of carbon in \[C{{H}_{3}}-Cl\]is
A)
\[-3\]
done
clear
B)
\[-2\]
done
clear
C)
\[-1\]
done
clear
D)
\[0\]
done
clear
View Answer play_arrow
question_answer 137) Which one of the following metals will not form an amalgam?
A)
Gold
done
clear
B)
Silver
done
clear
C)
Zinc
done
clear
D)
Iron
done
clear
View Answer play_arrow
question_answer 138) Incorrect statement regarding rusting is
A)
metallic iron is oxidised to \[F{{e}^{3+}}\]ions
done
clear
B)
metallic iron is reduced to \[F{{e}^{2-}}\] ions
done
clear
C)
oxygen gas is reduced to oxide ion
done
clear
D)
yellowish-brown product is formed
done
clear
View Answer play_arrow
question_answer 139) Which will be the proper alternative in place of A in the following equation? \[2F{{e}^{3+}}(aq)+S{{n}^{2+}}(aq)\xrightarrow{{}}2F{{e}^{2+}}(aq)+A\]
A)
\[S{{n}^{4+}}\]
done
clear
B)
\[S{{n}^{3+}}\]
done
clear
C)
\[S{{n}^{2+}}\]
done
clear
D)
\[S{{n}^{0}}\]
done
clear
View Answer play_arrow
question_answer 140) \[{{E}^{o}}=\frac{RT}{nF}\] In\[{{K}_{eq}},\] this equation is called
A)
Gibb's equation
done
clear
B)
Gibbs's-Helmholtz equation
done
clear
C)
Nernst equation
done
clear
D)
van der Waals' equation
done
clear
View Answer play_arrow
question_answer 141) Which of the following acts both as oxidising and reducing agent?
A)
\[HN{{O}_{3}}\]
done
clear
B)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\]
done
clear
C)
\[KMn{{O}_{4}}\]
done
clear
D)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 142) The oxidation number of oxygen in ozone is
A)
\[-3\]
done
clear
B)
\[-2\]
done
clear
C)
0 (zero)
done
clear
D)
\[+3\]
done
clear
View Answer play_arrow
question_answer 143) If an iron wire is placed in a solution of copper sulphate
A)
copper will precipitate out
done
clear
B)
iron will dissolve and precipitate out thereafter
done
clear
C)
iron and copper both will go into solution
done
clear
D)
no reaction will take place
done
clear
View Answer play_arrow
question_answer 144) To which of the following, the mass of an element deposited at an electrode is directly proportional?
A)
Atomic weight
done
clear
B)
Equivalent weight
done
clear
C)
Molecular weight
done
clear
D)
Atomic number
done
clear
View Answer play_arrow
question_answer 145) From the solution of which of the following one faraday of electricity will liberate one gram atom of metal?
A)
\[NaCl\]
done
clear
B)
\[BaC{{l}_{2}}\]
done
clear
C)
\[CuS{{O}_{4}}\]
done
clear
D)
\[AlC{{l}_{3}}\]
done
clear
View Answer play_arrow
question_answer 146) In which of the following, oxygen has an oxidation state of \[+2\]?
A)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
B)
\[C{{O}_{2}}\]
done
clear
C)
\[{{H}_{2}}O\]
done
clear
D)
\[O{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 147) Point out the false statement.
A)
Brownian movement and Tyndall effect is shown by colloidal systems
done
clear
B)
Gold number is a measure of the protective power of a lyophilic colloid
done
clear
C)
The colloidal solution of a liquid in liquid is called gel
done
clear
D)
Hardy-SchuIze rule is related with coagulation
done
clear
View Answer play_arrow
question_answer 148) Which one of the following will have the highest coagulating power for a ferric hydroxide sol?
A)
\[NaCl\]
done
clear
B)
\[BaC{{l}_{2}}\]
done
clear
C)
\[{{K}_{2}}Cr{{O}_{4}}\]
done
clear
D)
\[{{K}_{4}}[Fe{{(CN)}_{6}}]\]
done
clear
View Answer play_arrow
question_answer 149) Which of the following statement is false?
A)
A catalyst is specific in its action
done
clear
B)
A very small amount of the catalyst alters the rate of a reaction
done
clear
C)
The number of free valencies on the surface of the catalyst increases on subdivision
done
clear
D)
\[Ni\] is used as catalyst in the manufacture of ammonia
done
clear
View Answer play_arrow
question_answer 150) The natural materials from which an element can be extracted economically are called
A)
ores
done
clear
B)
minerals
done
clear
C)
Gangue
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 151) When a metal is to be extracted from its ore, if the gangue associated with the ore is silica, then
A)
An acidic flux is needed
done
clear
B)
a basic flux is needed
done
clear
C)
Both acidic and basic flux are needed
done
clear
D)
Neither of them is needed
done
clear
View Answer play_arrow
question_answer 152) An example of an oxide ore is
A)
Bauxite
done
clear
B)
malachite
done
clear
C)
Zinc blende
done
clear
D)
feldspar
done
clear
View Answer play_arrow
question_answer 153) In the Hoopes process, for refining of aluminium, the fused materials form three different layers and they remain separated during electrolysis also, this is because
A)
the upper layer is kept attracted by the cathode and the lower layer is kept attracted by the anode
done
clear
B)
There is special arrangement in the cell to keep the layers separate
done
clear
C)
the three layers have different densities
done
clear
D)
The three layers are maintained at different temperatures
done
clear
View Answer play_arrow
question_answer 154) In galvanisation, iron surface is coated with
A)
tin
done
clear
B)
chromium
done
clear
C)
zinc
done
clear
D)
nickel
done
clear
View Answer play_arrow
question_answer 155) In order to refine "Blister copper" it is melted in a furnace and is stirred with green logs of wood. The purpose is
A)
To expel the dissolved gases in blister copper
done
clear
B)
To bring the impurities to surface and oxidize them
done
clear
C)
To increase the carbon content of copper
done
clear
D)
To reduce the metallic oxide impurities with hydrocarbon gases liberated from the wood
done
clear
View Answer play_arrow
question_answer 156) On going from right to left in a period in the periodic table, the electronegativity of the elements
A)
Increases
done
clear
B)
Decreases
done
clear
C)
Remains unchanged
done
clear
D)
Decreases first then increases
done
clear
View Answer play_arrow
question_answer 157) In the periodic table, the element with atomic number 16 will be placed in the group
A)
third
done
clear
B)
fourth
done
clear
C)
fifth
done
clear
D)
sixth
done
clear
View Answer play_arrow
question_answer 158) Metals belonging to the same group in the periodic table are
A)
Magnesium and sodium
done
clear
B)
Magnesium and copper
done
clear
C)
Magnesium and barium
done
clear
D)
Magnesium and potassium
done
clear
View Answer play_arrow
question_answer 159) The electronic configuration in the outermost shell of halogens is
A)
\[{{s}^{2}}{{p}^{4}}\]
done
clear
B)
\[{{s}^{2}}{{p}^{5}}\]
done
clear
C)
\[{{s}^{2}}{{p}^{3}}\]
done
clear
D)
\[{{s}^{2}}{{p}^{6}}\]
done
clear
View Answer play_arrow
question_answer 160) The ionic radii \[{{N}^{3-}},\] \[{{O}^{2-}},\] \[{{F}^{-}}\]and \[N{{a}^{+}}\]follow the order
A)
\[{{N}^{3-}}>{{O}^{2-}}>{{F}^{-}}>N{{a}^{+}}\]
done
clear
B)
\[N{{a}^{3-}}>N{{a}^{+}}>{{O}^{2-}}>{{F}^{-}}\]
done
clear
C)
\[N{{a}^{+}}>{{O}^{2-}}>N{{a}^{3-}}>{{F}^{-}}\]
done
clear
D)
\[{{O}^{2-}}>{{F}^{-}}>N{{a}^{+}}>N{{a}^{3-}}\]
done
clear
View Answer play_arrow
question_answer 161) Which of the following electronic configurations is of a transition element?
A)
\[~1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}\]
done
clear
B)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{10}}4{{s}^{2}}4{{p}^{1}}\]
done
clear
C)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{p}^{6}}3{{d}^{10}}4{{s}^{2}}4{{p}^{6}}\]
done
clear
D)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{2}}4{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 162) Nessler's reagent is
A)
\[K{{H}_{g}}{{I}_{4}}\]
done
clear
B)
\[{{K}_{2}}Hg{{I}_{4}}+N{{H}_{4}}OH\]
done
clear
C)
\[{{K}_{2}}Hg{{I}_{4}}+KOH\]
done
clear
D)
\[KHg{{I}_{4}}+N{{H}_{4}}OH\]
done
clear
View Answer play_arrow
question_answer 163) Very dilute nitric acid reacts with zinc to form zinc nitrate and
A)
Ammonium nitrate
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[NO\]
done
clear
D)
\[{{N}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 164) By passing \[{{H}_{2}}S\] gas in acidified\[KMn{{O}_{4}}\]. solution, we get
A)
\[{{K}_{2}}S\]
done
clear
B)
\[S\]
done
clear
C)
\[{{K}_{2}}S{{O}_{3}}\]
done
clear
D)
\[Mn{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 165) If molecular weight of \[KMn{{O}_{4}}\] is ?M? then its equivalent weight in acidic medium would be
A)
\[M\]
done
clear
B)
\[M/2\]
done
clear
C)
\[M/5\]
done
clear
D)
\[M/4\]
done
clear
View Answer play_arrow
question_answer 166) \[Xe{{F}_{6}}\] on hydrolysis gives
A)
\[Xe{{O}_{3}}\]
done
clear
B)
\[XeO\]
done
clear
C)
\[Xe{{O}_{2}}\]
done
clear
D)
\[Xe\]
done
clear
View Answer play_arrow
question_answer 167) Which one of the following should be most stable?
A)
\[H_{2}^{+}\]
done
clear
B)
\[{{H}^{+}}\]
done
clear
C)
\[{{H}^{-}}\]
done
clear
D)
\[H\]
done
clear
View Answer play_arrow
question_answer 168) Molecular formula of Glauber's salt is
A)
\[MgS{{O}_{4}},7{{H}_{2}}O\]
done
clear
B)
\[CuS{{O}_{4}},5{{H}_{2}}O\]
done
clear
C)
\[FeS{{O}_{4}}.7{{H}_{2}}O\]
done
clear
D)
\[N{{a}_{2}}S{{O}_{4}}.10{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 169) The metal that is extracted from sea water is
A)
\[Mg\]
done
clear
B)
\[Ca\]
done
clear
C)
\[Fe\]
done
clear
D)
\[Ni\]
done
clear
View Answer play_arrow
question_answer 170) Which of the following oxides reacts with \[HCl\] and\[NaOH\]?
A)
\[CaO\]
done
clear
B)
\[ZnO\]
done
clear
C)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 171) The most basic element is
A)
fluorine
done
clear
B)
iodine
done
clear
C)
chlorine
done
clear
D)
bromine
done
clear
View Answer play_arrow
question_answer 172) An example of a double salt is
A)
Bleaching powder
done
clear
B)
Hypo
done
clear
C)
\[{{K}_{4}}{{[Fe(CN)]}_{6}}\]
done
clear
D)
Potash alum
done
clear
View Answer play_arrow
question_answer 173) Precipitate of \[AgCl\] dissolves in \[N{{H}_{4}}OH\] due to formation of a complex
A)
\[[Ag{{(N{{H}_{4}})}_{2}}]Cl\]
done
clear
B)
\[[Ag{{(N{{H}_{4}})}_{3}}]Cl\]
done
clear
C)
\[[Ag{{(N{{H}_{3}})}_{2}}]Cl\]
done
clear
D)
\[[Ag{{(N{{H}_{3}})}_{2}}OH]\]
done
clear
View Answer play_arrow
question_answer 174) The number of ions per mole of a complex \[[CoC{{l}_{2}}.{{(N{{H}_{3}})}_{4}}]Cl\] in aqueous solution will be
A)
nine
done
clear
B)
four
done
clear
C)
three
done
clear
D)
two
done
clear
View Answer play_arrow
question_answer 175) \[[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]N{{O}_{2}}\] and \[[Co{{(N{{H}_{3}})}_{4}}ClN{{O}_{2}}]Cl\] are isomers of which type?
A)
Geometrical
done
clear
B)
Optical
done
clear
C)
Linkage
done
clear
D)
Ionization
done
clear
View Answer play_arrow
question_answer 176) Which one of the following sulphides is only completely precipitated when the acidic solution is made dilute?
A)
\[HgS\]
done
clear
B)
\[PbS\]
done
clear
C)
\[CdS\]
done
clear
D)
\[CuS\]
done
clear
View Answer play_arrow
question_answer 177) Which of the following combines with \[F{{e}^{2+}}\] ions to form a brown complex?
A)
\[{{N}_{2}}O\]
done
clear
B)
\[NO\]
done
clear
C)
\[{{N}_{2}}{{O}_{3}}\]
done
clear
D)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 178) Which of the following substances consists of only one element?
A)
Marble
done
clear
B)
Sand
done
clear
C)
Diamond
done
clear
D)
Glass
done
clear
View Answer play_arrow
question_answer 179) An organic compound with \[C=40%\]and \[H=6.7%\]will have the empirical formula
A)
\[C{{H}_{2}}\]
done
clear
B)
\[C{{H}_{2}}O\]
done
clear
C)
\[{{C}_{3}}{{H}_{6}}{{O}_{3}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{4}}{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 180) The IUPAC name of the compound is \[C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{C}}\,}}\,H-C{{H}_{2}}-C{{H}_{2}}-Cl\]
A)
1-chloro-3-methylbutane
done
clear
B)
2-methyl-4-chlorobutane
done
clear
C)
2-methyl-1-chlorobutane
done
clear
D)
1-chloropentane
done
clear
View Answer play_arrow
question_answer 181) An organic compound will show optical isomerism if
A)
Four groups attached to C atom are different
done
clear
B)
Three groups attached to C atom are different
done
clear
C)
Two groups attached to C atom are different
done
clear
D)
All the groups attached to C atom are same
done
clear
View Answer play_arrow
question_answer 182) The arrangement of hydrogen atoms around carbon in the methane molecule is
A)
Triangular
done
clear
B)
Square planar
done
clear
C)
Tetrahedral
done
clear
D)
Hexagonal
done
clear
View Answer play_arrow
question_answer 183) The reaction between an Alkyl halide and sodium metal in dry ether is called
A)
Clemmensen reduction
done
clear
B)
Kolbe's reaction
done
clear
C)
Wurtz reaction
done
clear
D)
Cannizaro's reaction
done
clear
View Answer play_arrow
question_answer 184) Ethylene may be obtained by dehydration of which of the following with concentrated \[{{H}_{2}}S{{O}_{4}}\] at\[160-{{170}^{o}}C\]?
A)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
B)
\[C{{H}_{3}}OH\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}CHC{{H}_{2}}OH\]
done
clear
View Answer play_arrow
question_answer 185) Ethylene reacts with bromine to form
A)
\[Br-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}-C-B{{r}_{3}}\]
done
clear
C)
\[Br-C{{H}_{2}}-C{{H}_{2}}-Br\]
done
clear
D)
\[CHB{{r}_{3}}\]
done
clear
View Answer play_arrow
question_answer 186) The shape of methane molecule is
A)
Tetrahedral
done
clear
B)
Triangular
done
clear
C)
Planar
done
clear
D)
Octahedral
done
clear
View Answer play_arrow
question_answer 187) When chloroform is left open in light and air, it forms
A)
Methylene chloride
done
clear
B)
Formic acid
done
clear
C)
Carbon tetrachloride
done
clear
D)
Phosgene
done
clear
View Answer play_arrow
question_answer 188) Distinction is made between ethyl alcohol and methyl alcohol by
A)
Chloroform test
done
clear
B)
Victor Meyer test
done
clear
C)
Rate of esterification
done
clear
D)
Iodoform test
done
clear
View Answer play_arrow
question_answer 189) Chloroform on reduction with zinc and \[HCl\] forms
A)
Formic acid
done
clear
B)
Chloropicrin
done
clear
C)
Methyl chloride
done
clear
D)
Phosgene
done
clear
View Answer play_arrow
question_answer 190) From which of the following tertiary butyl alcohol is obtained by the action of methyl magnesium iodide?
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 191) For preparing an alkane, a concentrated aqueous solution of sodium or potassium salt of a saturated carboxylic acid is subjected to
A)
Hydrolysis
done
clear
B)
Oxidation
done
clear
C)
Hydrogenation
done
clear
D)
Electrolysis
done
clear
View Answer play_arrow
question_answer 192) If formaldehyde and \[KOH\]are heated, then we get
A)
Acetylene
done
clear
B)
Methane
done
clear
C)
Methyl alcohol
done
clear
D)
Ethyl formate
done
clear
View Answer play_arrow
question_answer 193) Which of the following reagent reacts differently with \[HCHO,\]\[C{{H}_{3}}CHO\] and\[C{{H}_{3}}COC{{H}_{3}}\]?
A)
\[HCN\]
done
clear
B)
\[N{{H}_{2}}N{{H}_{2}}\]
done
clear
C)
\[N{{H}_{2}}OH\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 194) Identify the product C in the series\[C{{H}_{3}}CN\xrightarrow{Na/{{C}_{2}}{{H}_{5}}OH}A\xrightarrow{HN{{O}_{2}}}B\]\[\xrightarrow{\begin{smallmatrix} Tollen's \\ reagent \end{smallmatrix}}C\]
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}NHOH\]
done
clear
C)
\[C{{H}_{3}}CON{{H}_{2}}\]
done
clear
D)
\[C{{H}_{3}}CHO\]
done
clear
View Answer play_arrow
question_answer 195) Lower carboxylic acid are soluble in-water due to
A)
Low molecular weight
done
clear
B)
Hydrogen bonding
done
clear
C)
Dissociation into ions
done
clear
D)
Easy hydrolysis
done
clear
View Answer play_arrow
question_answer 196) Ethyl acetate reacts with \[C{{H}_{3}}MgBr\]to form
A)
Secondary alcohol
done
clear
B)
Tertiary alcohol
done
clear
C)
Primary alcohol and acid
done
clear
D)
Acid
done
clear
View Answer play_arrow
question_answer 197) Which is used for the formation of nylon 66?
A)
Sulphur hexafluoride
done
clear
B)
Adipic acid
done
clear
C)
Sulphurous acid
done
clear
D)
Phthalic acid
done
clear
View Answer play_arrow
question_answer 198) Which of the following statements is false in the context of enzyme?
A)
Temperature affects their functioning
done
clear
B)
pH affects their functioning
done
clear
C)
They always increase activation energy
done
clear
D)
Their reactions are specific
done
clear
View Answer play_arrow
question_answer 199) Oils and fats together are called
A)
enzymes
done
clear
B)
soaps
done
clear
C)
polymer
done
clear
D)
lipids
done
clear
View Answer play_arrow
question_answer 200) The secondary structure of proteins is due to
A)
Peptide bond
done
clear
B)
Hydrogen bond
done
clear
C)
van der Waals' forces
done
clear
D)
Ionic bond
done
clear
View Answer play_arrow
question_answer 201) Whose secretion forms the peari?
A)
Prismatic layer
done
clear
B)
Columnar epithelial cells of mantle
done
clear
C)
Ciliated epithelial cells of mantle
done
clear
D)
Connective tissue of mantle
done
clear
View Answer play_arrow
question_answer 202) Striped muscles are present in
A)
lungs
done
clear
B)
gallbladder
done
clear
C)
blood vessels
done
clear
D)
limb muscles
done
clear
View Answer play_arrow
question_answer 203) The stored food material found in muscles is
A)
protein
done
clear
B)
glycogen
done
clear
C)
lipid
done
clear
D)
phosphogen
done
clear
View Answer play_arrow
question_answer 204) Striped muscles are
A)
syncytial
done
clear
B)
uninucleate
done
clear
C)
binucleate
done
clear
D)
anucleate
done
clear
View Answer play_arrow
question_answer 205) The main function of tendon is
A)
to join two bones
done
clear
B)
to join two muscles
done
clear
C)
to join muscles and bones
done
clear
D)
to join muscles and nerves
done
clear
View Answer play_arrow
question_answer 206) Nails are formed by
A)
bones
done
clear
B)
chitin
done
clear
C)
cartilage
done
clear
D)
keratin
done
clear
View Answer play_arrow
question_answer 207) In our body, the blood bank is
A)
red bone marrow
done
clear
B)
spleen
done
clear
C)
liver
done
clear
D)
heart
done
clear
View Answer play_arrow
question_answer 208) What type of poison is ethylene dichloride?
A)
Stomach poison
done
clear
B)
Contact poison
done
clear
C)
Fumigant
done
clear
D)
Biological control
done
clear
View Answer play_arrow
question_answer 209) Vocal cords are situated at
A)
pharynx
done
clear
B)
larynx
done
clear
C)
glottis
done
clear
D)
bronchial tube
done
clear
View Answer play_arrow
question_answer 210) Cutaneous glands are
A)
gastric glands
done
clear
B)
sebaceous glands
done
clear
C)
thyroid gland
done
clear
D)
thymus gland
done
clear
View Answer play_arrow
question_answer 211) Desmosome is the modification of
A)
Golgi bodies
done
clear
B)
endoplasmic membrane
done
clear
C)
plasma membrane
done
clear
D)
ER-nucleus complex
done
clear
View Answer play_arrow
question_answer 212) Bilirubin and biliverd in are present in
A)
pancreatic juice
done
clear
B)
saliva
done
clear
C)
bile juice
done
clear
D)
intestinal juice
done
clear
View Answer play_arrow
question_answer 213) Enzyme 'renin' is secreted by
A)
cells of stomach
done
clear
B)
cells of intestine
done
clear
C)
the cortical cells of kidney
done
clear
D)
the cells of juxtaglomerular apparatus of kidney
done
clear
View Answer play_arrow
question_answer 214) Dental formula of man is
A)
\[\frac{2123}{2123}\]
done
clear
B)
\[\frac{1223}{1223}\]
done
clear
C)
\[\frac{2132}{2132}\]
done
clear
D)
\[\frac{2213}{2213}\]
done
clear
View Answer play_arrow
question_answer 215) Muscles of alimentary canal are chiefly
A)
striated and neurogenic
done
clear
B)
un striated and neurogenic
done
clear
C)
striated and myogenic
done
clear
D)
un striated and myogenic
done
clear
View Answer play_arrow
question_answer 216) What is the total vital capacity of lungs in man?
A)
500 mL
done
clear
B)
2,000 mL
done
clear
C)
4,600 mL
done
clear
D)
5,800 mL
done
clear
View Answer play_arrow
question_answer 217) Loop of Henie is meant for absorption of
A)
potassium
done
clear
B)
glucose
done
clear
C)
water
done
clear
D)
\[C{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 218) In muscular contraction
A)
ATP breaks
done
clear
B)
ATP is formed
done
clear
C)
GTP breaks
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 219) pH of human blood is
A)
8.4
done
clear
B)
7.4
done
clear
C)
6.4
done
clear
D)
5.4
done
clear
View Answer play_arrow
question_answer 220) Oxygen is transported by
A)
blood plasma
done
clear
B)
leucocytes
done
clear
C)
RBC
done
clear
D)
thrombocytes
done
clear
View Answer play_arrow
question_answer 221) Lymph is colourless because
A)
WBCs are absent
done
clear
B)
WBCs are present
done
clear
C)
Haemoglobin is absent
done
clear
D)
RBCs are absent
done
clear
View Answer play_arrow
question_answer 222) Lymph (nodes) glands forms
A)
hormones
done
clear
B)
lymph
done
clear
C)
antigens
done
clear
D)
antibodies
done
clear
View Answer play_arrow
question_answer 223) In human beings, the function of spleen is
A)
to assist kidneys
done
clear
B)
to control blood pressure
done
clear
C)
to assist liver
done
clear
D)
to produce blood
done
clear
View Answer play_arrow
question_answer 224) Bowman's capsule is part of
A)
uriniferous tubule
done
clear
B)
renal artery
done
clear
C)
renal portal vein
done
clear
D)
ureter
done
clear
View Answer play_arrow
question_answer 225) Filtration takes place in
A)
Malpighian capsule
done
clear
B)
Bowman's capsule
done
clear
C)
glomerulus
done
clear
D)
collecting tubule
done
clear
View Answer play_arrow
question_answer 226) Main function of cerebellum is
A)
balancing
done
clear
B)
to see
done
clear
C)
to hear
done
clear
D)
remembering
done
clear
View Answer play_arrow
question_answer 227) Third ventricle is present in
A)
heart
done
clear
B)
brain
done
clear
C)
kidney
done
clear
D)
liver
done
clear
View Answer play_arrow
question_answer 228) The centre for sense of smell in brain is
A)
cerebellum
done
clear
B)
cerebrum
done
clear
C)
olfactory lobes
done
clear
D)
midbrain
done
clear
View Answer play_arrow
question_answer 229) The nerve related with diaphragm is
A)
vagus
done
clear
B)
phrenic
done
clear
C)
trigeminal
done
clear
D)
glossopharyngeal
done
clear
View Answer play_arrow
question_answer 230) Cowper's gland is present in
A)
cockroach
done
clear
B)
rabbit
done
clear
C)
earthworm
done
clear
D)
frog
done
clear
View Answer play_arrow
question_answer 231) Monoestrous animals are those which have
A)
one menses each month
done
clear
B)
one evularion each month
done
clear
C)
one egg
done
clear
D)
one breeding season in the year
done
clear
View Answer play_arrow
question_answer 232) For ovulation in reflex ovulators
A)
coitus is not necessary
done
clear
B)
coitus is necessary
done
clear
C)
plenty of food is necessary
done
clear
D)
plenty of food is not necessary
done
clear
View Answer play_arrow
question_answer 233) In sexually reproducing animals, the union of male and female gamete forms a cell which is called
A)
ovarian cell
done
clear
B)
oocyte
done
clear
C)
zygote
done
clear
D)
Graafian follicle
done
clear
View Answer play_arrow
question_answer 234) Fertilization occurs in
A)
ovary
done
clear
B)
fallopian tube
done
clear
C)
uterus
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 235) Quadriceps and gastrocnemius muscles lie in
A)
hands
done
clear
B)
legs
done
clear
C)
shoulder
done
clear
D)
wrist
done
clear
View Answer play_arrow
question_answer 236) The emergency hormone is
A)
thyroxin
done
clear
B)
adrenalin
done
clear
C)
insulin
done
clear
D)
progesterone
done
clear
View Answer play_arrow
question_answer 237) In a sperm, the mitochondria occur
A)
in tail
done
clear
B)
in acrosome
done
clear
C)
in middle piece
done
clear
D)
in head
done
clear
View Answer play_arrow
question_answer 238) Life-span of human RBC is
A)
120 days
done
clear
B)
90 days
done
clear
C)
2-3 days
done
clear
D)
20 days
done
clear
View Answer play_arrow
question_answer 239) Only rods are present in the eyes of one of the following animal
A)
pigeon
done
clear
B)
squirrel
done
clear
C)
fowl
done
clear
D)
owl
done
clear
View Answer play_arrow
question_answer 240) Pharynx and middle ear are interconnected by
A)
tympanic canal
done
clear
B)
eustachian canal
done
clear
C)
cochlear canal
done
clear
D)
vestibular canal
done
clear
View Answer play_arrow
question_answer 241) Proprioceptors refers to the ailment of
A)
chemicals
done
clear
B)
temperature
done
clear
C)
taste
done
clear
D)
change in the internal environment of the body
done
clear
View Answer play_arrow
question_answer 242) From outer to inside the sequence of three bones present in the middle ear of mammals is
A)
incus, malleus and stapes
done
clear
B)
malleus, incus and stapes
done
clear
C)
malleus, stapes and incus
done
clear
D)
stapes, malleus and incus
done
clear
View Answer play_arrow
question_answer 243) How many kinds of cells are found in islets Langerhans?
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 244) Diabetes insipidus is under the control of
A)
All dosterone
done
clear
B)
ADH
done
clear
C)
ACTH
done
clear
D)
TSH
done
clear
View Answer play_arrow
question_answer 245) FSH and LH hormones together are called
A)
emergency
done
clear
B)
gonadotropic hormones
done
clear
C)
neurohormones
done
clear
D)
outstress hormones
done
clear
View Answer play_arrow
question_answer 246) Parathormone deficiency in man causes
A)
hypercalcemia
done
clear
B)
hypocalcemia
done
clear
C)
goiter
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 247) Gorilla like man with large heads and protruding jaws, is produced due to
A)
over secretion of thyroxin
done
clear
B)
over secretion growth hormone since maturity
done
clear
C)
excess of vitamin-'C' in diet
done
clear
D)
excess secretion of TSH
done
clear
View Answer play_arrow
question_answer 248) Which one controls the secretion of estrogen
A)
HCG
done
clear
B)
Progesterone
done
clear
C)
LH
done
clear
D)
FSH
done
clear
View Answer play_arrow
question_answer 249) At cellular level, GH affects growth by controlling die production of
A)
rRNA
done
clear
B)
t-RNA
done
clear
C)
mRNA
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 250) Which of the following vitamins is essential for DNA synthesis and cell division ?
A)
Vitamin-A
done
clear
B)
Vitamin-D
done
clear
C)
Folic acid
done
clear
D)
Vitamin-K
done
clear
View Answer play_arrow
question_answer 251) Marasmum disease is caused due to
A)
protein deficiency
done
clear
B)
obesity
done
clear
C)
dwarfism
done
clear
D)
deficiency of vitamins
done
clear
View Answer play_arrow
question_answer 252) The brain develops from
A)
ectoderm
done
clear
B)
mesoderm
done
clear
C)
endoderm
done
clear
D)
meso-endoderm
done
clear
View Answer play_arrow
question_answer 253) The acrosome plays important role in
A)
motility of sperm
done
clear
B)
penetration of ovum by sperm
done
clear
C)
providing energy to sperm
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 254) The eggs of placental mammals are described as
A)
microlecithal
done
clear
B)
telolecithal
done
clear
C)
centrolecithal
done
clear
D)
macrolecithal
done
clear
View Answer play_arrow
question_answer 255) Oogenesis is the process for the formation of
A)
red blood cells
done
clear
B)
sperms
done
clear
C)
ova
done
clear
D)
sperms and ova
done
clear
View Answer play_arrow
question_answer 256) The third phase in the development of mammal is
A)
cleavage
done
clear
B)
gastrulation
done
clear
C)
gametogenesis
done
clear
D)
fertilization
done
clear
View Answer play_arrow
question_answer 257) During ageing, collagen present in intercellular spaces becomes
A)
Destroyed
done
clear
B)
Impremeable and rigid
done
clear
C)
More elastic
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 258) The theory of ageing holds that ageing is due to
A)
random mutation in DNA of somatic cells
done
clear
B)
increased cross-linkage of collagen and other proteins
done
clear
C)
cumulative result of damage to tissues by free radicals
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 259) If mother has blood group B, father has A group, the offspring will be of
A)
A group
done
clear
B)
O group
done
clear
C)
Any of the group
done
clear
D)
AB group
done
clear
View Answer play_arrow
question_answer 260) Erythroblastosis foetalis occurs when
A)
husband is \[R{{h}^{-}}\] and wife \[R{{h}^{-}}\]
done
clear
B)
wife is \[R{{h}^{-}}\]and husband \[R{{h}^{-}}\]
done
clear
C)
wife is \[R{{h}^{-}}\]and husband \[R{{h}^{+}}\]
done
clear
D)
wife is \[R{{h}^{-}}\]and husband \[R{{h}^{+}}\]
done
clear
View Answer play_arrow
question_answer 261)
Match List \[\text{I}\] with List \[\text{II}\] and select the correct answer using the codes given below List \[\text{I}\] (Inheritable disease) List\[\text{II}\] (Symptoms) (A) Achondroplasia 1. Blood coagulation delayed due to absence of factor IX (B) Cystinuria 2. Darkness due to short limb bones, head is normal (C) Epilepsy 3. Periodic convulsions and unconsciousness (D) Christmas disease 4. Renal stone formation due to excessive excretion of cytine in urine
Codes:
A)
A-1 A-2 A-3 A-4
done
clear
B)
B-2 B-4 B-3 B-1
done
clear
C)
C-4 C-3 C-2 C-1
done
clear
D)
D-3 D-4 D-1 D-2
done
clear
View Answer play_arrow
question_answer 262) The frequency of mutant gene in a population is expected to increase if that gene is
A)
dominant
done
clear
B)
recessive
done
clear
C)
sex linked
done
clear
D)
favourably selected
done
clear
View Answer play_arrow
question_answer 263) Abzymes are
A)
abnormal enzymes
done
clear
B)
enzymes act on antibodies
done
clear
C)
antibodies act as enzymes
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 264) Whereas the number of chromosomes is reduced to half in first reduction division of meiosis, then what is the need for second mitotic division?
A)
For the segregation of replicated chromosomes
done
clear
B)
For equal distribution of haploid chromosomes
done
clear
C)
For the formation of four gametes
done
clear
D)
For equal distribution of genes on chromosomes
done
clear
View Answer play_arrow
question_answer 265) During systole
A)
auricles and ventricles contract simultaneously
done
clear
B)
auricles and ventricles contract separately
done
clear
C)
only auricles contract
done
clear
D)
only ventricles contract
done
clear
View Answer play_arrow
question_answer 266) Hybridoma technology was developed by
A)
Taggart; 1982
done
clear
B)
Prie and Saxton; 1987
done
clear
C)
Vitella et al; 1982
done
clear
D)
Kohler and Milstein; 1982
done
clear
View Answer play_arrow
question_answer 267) In F2 generation, quantitative inheritance 1:4: 6 : 4 : 1 is obtained instead of
A)
8 : 6 : 4 : 1
done
clear
B)
7 : 4 :1: 4
done
clear
C)
9 : 3 : 3 : 1
done
clear
D)
6 : 6 : 4 : 7
done
clear
View Answer play_arrow
question_answer 268) Acromion process is found in rabbit in
A)
pectoral girdle
done
clear
B)
femur
done
clear
C)
vertebral column
done
clear
D)
pelvic girdle
done
clear
View Answer play_arrow
question_answer 269) The most, commonly used enzyme for polymerase chain reaction is
A)
DNA polymerase\[\text{-II}\]
done
clear
B)
Reverse transcriptase
done
clear
C)
Klenow fragment
done
clear
D)
Taq polymerase
done
clear
View Answer play_arrow
question_answer 270) In humans, sex determination of the offspring is decided by the
A)
sex chromosome of mother
done
clear
B)
sex chromosome of father
done
clear
C)
size of ovum
done
clear
D)
size of sperm
done
clear
View Answer play_arrow
question_answer 271) If somatic cells of a human male contain single Barr body, the genetic composition of the person would be
A)
XYY
done
clear
B)
XXY
done
clear
C)
XO
done
clear
D)
XXXY
done
clear
View Answer play_arrow
question_answer 272) The main cause of paralysis is
A)
some defect in muscles
done
clear
B)
complete destruction of sensory nerves
done
clear
C)
complete destruction of motor nerves
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 273) Sex ratio in a species indicates the numerical relation between the number of
A)
male and female producing gametes
done
clear
B)
blacks and whites
done
clear
C)
unisexual and bisexual
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 274) Systema naturae is concerned with
A)
solar system
done
clear
B)
ecosystem
done
clear
C)
classification of plants and animals
done
clear
D)
natural selection
done
clear
View Answer play_arrow
question_answer 275) Phylogeny tells about
A)
life history of animals
done
clear
B)
group of phyla
done
clear
C)
evolutionary history of a species from its ancestors
done
clear
D)
castes of flies
done
clear
View Answer play_arrow
question_answer 276) Which of the following classes has largest number of animals?
A)
Mammalia
done
clear
B)
Pisces
done
clear
C)
Insecta
done
clear
D)
Reptilia
done
clear
View Answer play_arrow
question_answer 277) Cray fish is a
A)
crustacean animal
done
clear
B)
edible fish
done
clear
C)
poisonous fish
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 278) Which of the following is not found in vertebrates?
A)
Bilateral symmetry
done
clear
B)
Gill opening
done
clear
C)
Body scales
done
clear
D)
Cnidoblasts
done
clear
View Answer play_arrow
question_answer 279) A species defined as "the group of actually or potentially inter-breeding natural population producing fertile offspring and reproductive isolated from other groups" The above statement is given by
A)
Carolus Linnaeus
done
clear
B)
Mayr
done
clear
C)
J B Lamarck
done
clear
D)
Charles Darwin
done
clear
View Answer play_arrow
question_answer 280) Origin of life took place in/on
A)
water
done
clear
B)
air
done
clear
C)
mountains
done
clear
D)
land
done
clear
View Answer play_arrow
question_answer 281) Homologous organs are
A)
similar in origin
done
clear
B)
similar in function
done
clear
C)
similar in development
done
clear
D)
similar in behavior
done
clear
View Answer play_arrow
question_answer 282) Vestigial organs are those organs, which are
A)
characteristic of birds
done
clear
B)
not of much use today
done
clear
C)
helpful in locomotion
done
clear
D)
common
done
clear
View Answer play_arrow
question_answer 283) Lamarckian theory deals with
A)
acquired characters
done
clear
B)
germplasm
done
clear
C)
struggle for existence
done
clear
D)
mutation
done
clear
View Answer play_arrow
question_answer 284) A species is produced by loss or disappearance of a few characters founds in parent. It is termed as
A)
progressive species
done
clear
B)
retrogressive species
done
clear
C)
successive species
done
clear
D)
digressive species
done
clear
View Answer play_arrow
question_answer 285) Isinglass, a type of byproduct of fish industry is principally used for
A)
feeding catle, pigs and poultry
done
clear
B)
preparation of paints and varnishes
done
clear
C)
clarification of vinegar, wines and beer
done
clear
D)
production of insulin
done
clear
View Answer play_arrow
question_answer 286) Which one of the following combination is generally recommended for composite fish farming in India?
A)
Caria, Cyprinus, Clarias
done
clear
B)
Caria, Labeo, Cirrhinus
done
clear
C)
Cirrhinus, Cyprinus, Channa
done
clear
D)
Clarias, Chanos, Cyprinus
done
clear
View Answer play_arrow
question_answer 287) The indigenous adult fish, which may be used most effectively for the biological control of mosquitoes is
A)
Aplocheilus
done
clear
B)
Gambusia
done
clear
C)
Legistes
done
clear
D)
Caria
done
clear
View Answer play_arrow
question_answer 288) The disease 'Oriental sore' is caused by
A)
bacteria
done
clear
B)
virus
done
clear
C)
Protozoa
done
clear
D)
fungus
done
clear
View Answer play_arrow
question_answer 289) The carrier of Trypanosoma in man is
A)
house fly
done
clear
B)
mosquito
done
clear
C)
honey bee
done
clear
D)
tse tse fly
done
clear
View Answer play_arrow
question_answer 290) The carrier of virus causing human yellow fever is
A)
mosquito
done
clear
B)
bug
done
clear
C)
louse
done
clear
D)
beetle
done
clear
View Answer play_arrow
question_answer 291) Which of the following is the cancerous state of blood?
A)
Chloremia
done
clear
B)
Leukaemia
done
clear
C)
Uremia
done
clear
D)
Proteinemia
done
clear
View Answer play_arrow
question_answer 292) Against, which of the following does interferon act?
A)
Bacteria
done
clear
B)
Virus
done
clear
C)
Fungus
done
clear
D)
Snake venom
done
clear
View Answer play_arrow
question_answer 293) Which of the following is a sexually transmitted disease?
A)
Malaria
done
clear
B)
Syphilis
done
clear
C)
Goiter
done
clear
D)
Tuberculosis
done
clear
View Answer play_arrow
question_answer 294) Jaundice is caused by
A)
contaminated water
done
clear
B)
pork
done
clear
C)
excessive sugar
done
clear
D)
excessive eating of Curcuma
done
clear
View Answer play_arrow
question_answer 295) AIDS can be transmitted by
A)
blood circulation
done
clear
B)
handshake
done
clear
C)
courtship
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 296) Smoking addiction is harmful because it produces polycyclic aromatic hydrocarbons which cause
A)
reduction in oxygen transport
done
clear
B)
increase in blood pressure
done
clear
C)
cancer
done
clear
D)
retardation of growth of foetus
done
clear
View Answer play_arrow
question_answer 297) Alcohol addiction is harmful because it causes
A)
protein depositions in liver
done
clear
B)
deposition of extra fat in liver
done
clear
C)
rise in blood sugar level
done
clear
D)
cancer
done
clear
View Answer play_arrow
question_answer 298) Dudhawa national park is located in
A)
Madhya Pradesh
done
clear
B)
Himachal Pradesh
done
clear
C)
Arunachal Pradesh
done
clear
D)
Uttar Pradesh
done
clear
View Answer play_arrow
question_answer 299) BHC and DDT belong to which class of pesticides?
A)
Organophosphates
done
clear
B)
Organochlorines
done
clear
C)
Carbonates
done
clear
D)
Triazines
done
clear
View Answer play_arrow
question_answer 300) All chordates at one or the other stage have
A)
pharyngeal gill slits
done
clear
B)
vertebral column
done
clear
C)
two pairs of pentadacyle limb
done
clear
D)
a movable jaw
done
clear
View Answer play_arrow
question_answer 301) Which is not character of mitosis?
A)
Leptotene
done
clear
B)
Zygotene
done
clear
C)
Pachytene
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 302) Who called the living matter of the cell as protoplasm?
A)
Purkinje
done
clear
B)
Virchow
done
clear
C)
Hooke
done
clear
D)
Khorana
done
clear
View Answer play_arrow
question_answer 303) How many types of cells are known?
A)
One
done
clear
B)
Two
done
clear
C)
Four
done
clear
D)
Five
done
clear
View Answer play_arrow
question_answer 304) In which, mitosis does not occur?
A)
Green algae
done
clear
B)
Fungi
done
clear
C)
Bacteria
done
clear
D)
higher plants
done
clear
View Answer play_arrow
question_answer 305) A mature plant cell has
A)
cell wall
done
clear
B)
vacuole
done
clear
C)
protoplasm
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 306) In which, plastids are not found?
A)
Blue-green algae
done
clear
B)
Bacteria
done
clear
C)
Fungi
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 307) Repulsion of homologous chromosomes takes place in
A)
zygotene
done
clear
B)
leptotene
done
clear
C)
diakinesis
done
clear
D)
diplotene
done
clear
View Answer play_arrow
question_answer 308) In eukaryotic cell, the type of ribosome is
A)
Only 70S
done
clear
B)
Only 80S
done
clear
C)
Both [a] and [b]
done
clear
D)
Only SOS
done
clear
View Answer play_arrow
question_answer 309) Synthesis of DNA takes place in
A)
\[{{G}_{1}}\]
done
clear
B)
\[~{{G}_{2}}\]
done
clear
C)
S
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 310) The genetic material of prokaryotic cells is called
A)
nucleus
done
clear
B)
nucleolus
done
clear
C)
nucleoid
done
clear
D)
centrosome
done
clear
View Answer play_arrow
question_answer 311) Which organelle of plant secretes polysaccharide and protein to make cell walls?
A)
Golgi bodies
done
clear
B)
Lysosome
done
clear
C)
Mitochondria
done
clear
D)
Chloroplast
done
clear
View Answer play_arrow
question_answer 312) RNA contains which of the following base, in place of thymine of DNA?
A)
Thymine
done
clear
B)
Uracil
done
clear
C)
Adenine
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 313) The main function of lysosome is in
A)
only extracellular digestion
done
clear
B)
only intracellular digestion
done
clear
C)
Both [a] and [d]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 314) Eukaryotic cell has
A)
chromatin fibre
done
clear
B)
definite nucleus
done
clear
C)
incipient nucleus
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 315) At which stage of mitosis, chromatids separse and start moving towards poles?
A)
Prophase
done
clear
B)
Metaphase
done
clear
C)
Anaphase
done
clear
D)
Telophase
done
clear
View Answer play_arrow
question_answer 316) TMV contains
A)
DNA
done
clear
B)
RNA + Protein
done
clear
C)
DNA + RNA
done
clear
D)
DNA + Protein
done
clear
View Answer play_arrow
question_answer 317) Four daughter cells formed after meiosis are
A)
genetically similar
done
clear
B)
genetically different
done
clear
C)
anucleate
done
clear
D)
multinucleate
done
clear
View Answer play_arrow
question_answer 318) The synthesis of lipids and lipoproteins is associated with
A)
endoplasmic reticulum
done
clear
B)
mitochondria
done
clear
C)
chloroplast
done
clear
D)
lysosomes
done
clear
View Answer play_arrow
question_answer 319) DNA strands are and parallel because
A)
H-bonds
done
clear
B)
phosphate diester bonds
done
clear
C)
disulphide bonds
done
clear
D)
phosphate bonds
done
clear
View Answer play_arrow
question_answer 320) Cell theory was proposed by
A)
Schleiden and Schwann
done
clear
B)
Waston and Crick
done
clear
C)
Darwin and Wallace
done
clear
D)
Mendel and Morgan
done
clear
View Answer play_arrow
question_answer 321) During meiosis the division of centromere takes place in
A)
first prophase
done
clear
B)
first anaphase
done
clear
C)
second metaphase
done
clear
D)
second anaphase
done
clear
View Answer play_arrow
question_answer 322) Which one of the following is not found in animal cell?
A)
Nucleus
done
clear
B)
Golgi bodies
done
clear
C)
Chloroplast
done
clear
D)
Mitochondria
done
clear
View Answer play_arrow
question_answer 323) Unit membrane consists of
A)
Lipid + Sugar + Protein
done
clear
B)
Protein + Lipid + Protein
done
clear
C)
Lipid + Protein + Lipid
done
clear
D)
Protein
done
clear
View Answer play_arrow
question_answer 324) Translation is the process, in which
A)
DNA is formed on DNA template
done
clear
B)
RNA is formed on DNA template
done
clear
C)
DNA is formed on RNA template
done
clear
D)
Protein is formed from mRNA
done
clear
View Answer play_arrow
question_answer 325) Linkage was first studied by
A)
Darwin
done
clear
B)
Morgan
done
clear
C)
Bateson and Punned
done
clear
D)
Mendel
done
clear
View Answer play_arrow
question_answer 326) Principal constituents of chromosomes are
A)
DNA
done
clear
B)
RNA
done
clear
C)
Both [a] and [b]
done
clear
D)
tRNA
done
clear
View Answer play_arrow
question_answer 327) Who explained the Operon model for the first time?
A)
Francois Jacob
done
clear
B)
Jacques Monod
done
clear
C)
Both [a] and [b]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 328) The enzyme responsible for transcription is
A)
DNA polymerase-I
done
clear
B)
RNA polymerase
done
clear
C)
Reverse transcriptase
done
clear
D)
DNA polymerase-III
done
clear
View Answer play_arrow
question_answer 329) Initiation codon is
A)
AUG
done
clear
B)
UAG
done
clear
C)
UGA
done
clear
D)
UAA
done
clear
View Answer play_arrow
question_answer 330) The direction of DNA replication is
A)
from 5' end towards 3' end
done
clear
B)
from 3' end towards 5' end
done
clear
C)
amino terminus to carboxy terminus
done
clear
D)
carboxy terminus to amino terminus
done
clear
View Answer play_arrow
question_answer 331) Extra chromosomal genetic material found in bacteria is
A)
plasmid
done
clear
B)
micro some
done
clear
C)
ribosome
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 332) Functional unit of gene is
A)
muton
done
clear
B)
recon
done
clear
C)
cistron
done
clear
D)
codon
done
clear
View Answer play_arrow
question_answer 333) A mutant micro-organism unable to synthesize a compound required for its growth but able to grow if the compound is provided, is known as
A)
auxotroph
done
clear
B)
prototroph
done
clear
C)
autotroph
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 334) Incomplete dominance is found in
A)
Pisum sativum
done
clear
B)
Antirrhinum majus
done
clear
C)
Both [a] and [b]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 335) Shape of chromosome is determined by
A)
telomere
done
clear
B)
centromere
done
clear
C)
chromomere
done
clear
D)
centrosome
done
clear
View Answer play_arrow
question_answer 336) How many types of genotypes are formed in \[{{F}_{2}}\] progeny obtained from self pollination of a dihybrid \[{{F}_{1}}\]?
A)
9
done
clear
B)
3
done
clear
C)
6
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 337) Functioning of structural genes is controlled by
A)
operator
done
clear
B)
promoter
done
clear
C)
ligase
done
clear
D)
regulatory gene
done
clear
View Answer play_arrow
question_answer 338) Domestic cooking gas cylinder is filled with
A)
alcohol
done
clear
B)
diesel oil
done
clear
C)
liquid petroleum gas
done
clear
D)
coal gas
done
clear
View Answer play_arrow
question_answer 339) Sometimes when a virus attacks a bacterium, neither the virus multiplies nor the bacterium does. This phenomenon is called
A)
adsorption
done
clear
B)
assimilation
done
clear
C)
lysogeny
done
clear
D)
viral stability
done
clear
View Answer play_arrow
question_answer 340) An organism having cytoplasm, DNA and RNA but no cell wall is
A)
cyano bacterium
done
clear
B)
mycoplasma
done
clear
C)
bacterium
done
clear
D)
virus
done
clear
View Answer play_arrow
question_answer 341) Among the following which are the smallest organisms?
A)
Bacteria
done
clear
B)
Mycoplasma
done
clear
C)
Nostoc
done
clear
D)
Virus
done
clear
View Answer play_arrow
question_answer 342) The genetic material in viruses is
A)
in some viruses both DNA and RNA
done
clear
B)
only RNA
done
clear
C)
only DNA
done
clear
D)
in some viruses only DNA and in some only RNA
done
clear
View Answer play_arrow
question_answer 343) One of the useful activities of several bacteria is
A)
nitrogen fixation
done
clear
B)
nitrification
done
clear
C)
operation of biogeochemical cycles
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 344) In a bacterial cell, the organelle with respiratory enzymes is
A)
mitochondria
done
clear
B)
chondrisome
done
clear
C)
mesosome
done
clear
D)
centrosome
done
clear
View Answer play_arrow
question_answer 345) Circular DNA molecule occurs in
A)
viruses
done
clear
B)
bacteria, chloroplasts and mitochondria
done
clear
C)
bacteria and chloroplasts only
done
clear
D)
bacteria only
done
clear
View Answer play_arrow
question_answer 346) A bacteriophage is a
A)
virus attacking another virus
done
clear
B)
stage in the life cycle of a bacterium
done
clear
C)
bacterium attacking a virus
done
clear
D)
virus attacking a bacterium
done
clear
View Answer play_arrow
question_answer 347) The cells of the bacterium Streptococcus remain arranged in the form of
A)
chain
done
clear
B)
irregular cluster
done
clear
C)
cube
done
clear
D)
plate
done
clear
View Answer play_arrow
question_answer 348) The main function of elementary bodies in mycoplasma is
A)
reproduction
done
clear
B)
nutrition
done
clear
C)
secretion
done
clear
D)
respiration
done
clear
View Answer play_arrow
question_answer 349) The water of Holy Gang as river is pure due to the presence of
A)
cyanophages
done
clear
B)
hydrophytes
done
clear
C)
bacteria
done
clear
D)
bacteriophages
done
clear
View Answer play_arrow
question_answer 350) Which one of the following can utilize molecular nitrogen \[({{N}_{2}})\] as nutrient for growth?
A)
Khizobium
done
clear
B)
Spirogyra
done
clear
C)
Mucor
done
clear
D)
Methanococcus
done
clear
View Answer play_arrow
question_answer 351) In Pinus, male and female reproductive structures occur?
A)
On different branches of the same plant
done
clear
B)
On different plants
done
clear
C)
On same branch
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 352) The cell wall of Spirogyra is made up of
A)
cellulose
done
clear
B)
pectin
done
clear
C)
lignin
done
clear
D)
chitin
done
clear
View Answer play_arrow
question_answer 353) Stomata in Funaria are found in
A)
capsule
done
clear
B)
foot
done
clear
C)
seta
done
clear
D)
nowhere
done
clear
View Answer play_arrow
question_answer 354) A bisexual flower, which never opens in life is known as
A)
homogamous
done
clear
B)
heterogamous
done
clear
C)
dichogamous
done
clear
D)
cleistogamous
done
clear
View Answer play_arrow
question_answer 355) Eichhornia crassipes is a
A)
desert plant
done
clear
B)
parasite
done
clear
C)
water plant
done
clear
D)
terrestrial plant
done
clear
View Answer play_arrow
question_answer 356) Plants of salty seashore wet-lands are call?
A)
halophytes
done
clear
B)
heliophytes
done
clear
C)
hydrophytes
done
clear
D)
saprophytes
done
clear
View Answer play_arrow
question_answer 357) If testa is removed from water soaked gram seed, the remaining structure is
A)
full mature embryo
done
clear
B)
cotyledons with endosperm and perican
done
clear
C)
cotyledons filled with starch
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 358) Double fertilization means fusion of
A)
one of the polar nuclei with the other one male gamete with egg cell
done
clear
B)
one of the male gamete with polar nuclei and other with antipodal cells
done
clear
C)
one of the male gamete with secondary nucleus and the other male gamete with egg cell
done
clear
D)
one antipodal cell with egg cell and synergid cell with antipodal cell
done
clear
View Answer play_arrow
question_answer 359) Double fertilization was first discovered by Nawaschin (1898) in
A)
Lilium and Fritillaria
done
clear
B)
mango and sugarcane
done
clear
C)
papaya and pea
done
clear
D)
Brassica and candytuft
done
clear
View Answer play_arrow
question_answer 360) In an angiospermic plant, endosperm is formed due to fertilization of secondary nucleus but is absent in some of the seeds viz pea bean Phaseolus (moong) etc. It is due to lack of
A)
certain enzymes
done
clear
B)
dicotyledonous hormone
done
clear
C)
growth hormone
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 361) A component of xylem is
A)
sieve tube
done
clear
B)
medullary ray
done
clear
C)
sclereid
done
clear
D)
tracheid
done
clear
View Answer play_arrow
question_answer 362) A group of cells alike in forms, function and origin is called
A)
organ
done
clear
B)
organelle
done
clear
C)
tissue
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 363) Hard, lignified, thick walled, long and pointed cells in a plant are
A)
parenchyma
done
clear
B)
sclerenchyma
done
clear
C)
collenchyma
done
clear
D)
sclereids
done
clear
View Answer play_arrow
question_answer 364) The meristem, which develops into a primary vascular tissue is
A)
protonema
done
clear
B)
promeristem
done
clear
C)
ground meristem
done
clear
D)
procambium
done
clear
View Answer play_arrow
question_answer 365) Which one of the following elements is required for photosynthesis oxygen evolution?
A)
Copper
done
clear
B)
Iron
done
clear
C)
Manganese
done
clear
D)
Zinc
done
clear
View Answer play_arrow
question_answer 366) Sinks are related to
A)
transport of organic solutes
done
clear
B)
stomata
done
clear
C)
enzymes
done
clear
D)
phytochrome
done
clear
View Answer play_arrow
question_answer 367) Supply ends in transport of solute are
A)
green leaves and storage organs
done
clear
B)
root and stem
done
clear
C)
xylem and phloem
done
clear
D)
Hormones and enzymes
done
clear
View Answer play_arrow
question_answer 368) Stomata can open at night also in
A)
xerophyte
done
clear
B)
gametophyte
done
clear
C)
hydrophyte
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 369) Who had said that 'transpiration is a necessary evil'?
A)
Curtis
done
clear
B)
Steward
done
clear
C)
Anderson
done
clear
D)
J C Bose
done
clear
View Answer play_arrow
question_answer 370) Agranal chloroplasts occur in certain
A)
succulents
done
clear
B)
\[{{C}_{3}}-\]plants
done
clear
C)
\[{{C}_{4}}-\]plants
done
clear
D)
hydrophytes
done
clear
View Answer play_arrow
question_answer 371) The wavelength of light most absorbed during photosynthesis is
A)
400nm
done
clear
B)
550nm
done
clear
C)
660nm
done
clear
D)
70nm
done
clear
View Answer play_arrow
question_answer 372) Which are related with photorespiration?
A)
Spherosome
done
clear
B)
Lysosomes
done
clear
C)
Glyoxysomes
done
clear
D)
Peroxisomes
done
clear
View Answer play_arrow
question_answer 373) The end product of glycolysis is
A)
glucose
done
clear
B)
fructose
done
clear
C)
pyruvic acid
done
clear
D)
ethyl alcohol
done
clear
View Answer play_arrow
question_answer 374) RQ is more than one in case of
A)
fat
done
clear
B)
fructose
done
clear
C)
glucose
done
clear
D)
organic add
done
clear
View Answer play_arrow
question_answer 375) Enzymes concerned with transfer of electrons are
A)
hydrolysases
done
clear
B)
dehydrogenases
done
clear
C)
transminases
done
clear
D)
proteases
done
clear
View Answer play_arrow
question_answer 376) Nitrogen is the essential component of
A)
carbohydrates
done
clear
B)
fats
done
clear
C)
proteins
done
clear
D)
oil
done
clear
View Answer play_arrow
question_answer 377) Phytochrome was discovered by
A)
W Went
done
clear
B)
Gamer and Allard
done
clear
C)
F F Blackman
done
clear
D)
R Hill
done
clear
View Answer play_arrow
question_answer 378) The activity of a-amylase in the endospore of barley germinating seed is induced by
A)
ethylene
done
clear
B)
cytokinin
done
clear
C)
IAA
done
clear
D)
gibberellins
done
clear
View Answer play_arrow
question_answer 379) A pigment concerned with both floral induction and seed germination is
A)
florigen
done
clear
B)
chlorophyll
done
clear
C)
plastocyanin
done
clear
D)
phytochrome
done
clear
View Answer play_arrow
question_answer 380) Name the famous plant ecologist
A)
Jagdish Chandra Bose
done
clear
B)
Birbal Sahani
done
clear
C)
Ramdeva Mishra
done
clear
D)
Charles Darwin
done
clear
View Answer play_arrow
question_answer 381) Possibilities of presence of coal in a particular area can be guessed by the study of
A)
Economic botany
done
clear
B)
Ecology
done
clear
C)
pollen grain analysis
done
clear
D)
mining the area
done
clear
View Answer play_arrow
question_answer 382) The significance of ecosystem lies in
A)
flow of energy
done
clear
B)
cycling of matter
done
clear
C)
Both [a] and [b]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 383) Biological equilibrium is found among the
A)
producers and consumers
done
clear
B)
producers and decomposers
done
clear
C)
producers, consumers and decomposers
done
clear
D)
producers and light
done
clear
View Answer play_arrow
question_answer 384) The number of individual of a species in a particular ecosystem at a given time remains constant due to
A)
man
done
clear
B)
parasites
done
clear
C)
predators
done
clear
D)
available food
done
clear
View Answer play_arrow
question_answer 385) The two components of an ecosystem are
A)
biotic and abiotic
done
clear
B)
plants and animals
done
clear
C)
weeds and micro-organisms
done
clear
D)
plants and light
done
clear
View Answer play_arrow
question_answer 386) The pyramid of number in a grassland ecosystem is
A)
linear
done
clear
B)
upright
done
clear
C)
irregular
done
clear
D)
inverted
done
clear
View Answer play_arrow
question_answer 387) One of the important effects of \[S{{O}_{2}}\] and its transformation products on plants is
A)
plasmolysis
done
clear
B)
destruction of chlorophyll
done
clear
C)
destruction of Golgi bodies
done
clear
D)
destruction of cell wall
done
clear
View Answer play_arrow
question_answer 388) Major pollutant present in the jet plane emission is
A)
carbon tetrachloride
done
clear
B)
sulphur dioxide
done
clear
C)
carbon monoxide
done
clear
D)
fluorocarbon
done
clear
View Answer play_arrow
question_answer 389) Sewage water is purified for recycling by the action of
A)
light
done
clear
B)
micro-organisms
done
clear
C)
aquatic plants
done
clear
D)
fishes
done
clear
View Answer play_arrow
question_answer 390) Spraying of DDT on crops produces pollution of
A)
soil and water
done
clear
B)
air and soil
done
clear
C)
crops and air
done
clear
D)
air and water
done
clear
View Answer play_arrow
question_answer 391) Which one is not dangerous
A)
bio pollutants
done
clear
B)
ozone layer
done
clear
C)
nuclear blast
done
clear
D)
deforestation
done
clear
View Answer play_arrow
question_answer 392) A drug from root, which causes mental disorder and reduces blood pressure is obtained from
A)
Rauwolfia serpentine
done
clear
B)
Atropa baladona
done
clear
C)
Colchicum luteum
done
clear
D)
Digitalis purpurea
done
clear
View Answer play_arrow
question_answer 393) Maximum sugarcane grown in
A)
Madhya Pradesh
done
clear
B)
Bihar
done
clear
C)
Punjab
done
clear
D)
Uttar Pradesh
done
clear
View Answer play_arrow
question_answer 394) Which one is the riches source of vegetarian protein?
A)
Gram
done
clear
B)
Soyabean
done
clear
C)
Wheat
done
clear
D)
Rice
done
clear
View Answer play_arrow
question_answer 395) Branch of Botany connected with study of food, fibre and wood yielding plant
A)
Ethnobotany
done
clear
B)
Palaeobotany
done
clear
C)
Economic botany
done
clear
D)
Microbiology
done
clear
View Answer play_arrow
question_answer 396) Which fibre crop occupies the maximum area in India?
A)
Jute
done
clear
B)
Cotton
done
clear
C)
Flax
done
clear
D)
Sisal
done
clear
View Answer play_arrow
question_answer 397) Botanical name of tea is
A)
Piper nigrum
done
clear
B)
Thea/Camellia sinensis
done
clear
C)
Coffea Arabica
done
clear
D)
Tectona grandis
done
clear
View Answer play_arrow
question_answer 398) Which can preserve food stuffs?
A)
Salt and sugar
done
clear
B)
Sugar and vinegar
done
clear
C)
Vinegar
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 399) Forest research institute is situated at
A)
Lucknow
done
clear
B)
Bhopal
done
clear
C)
Hyderabad
done
clear
D)
Dehradun
done
clear
View Answer play_arrow
question_answer 400) Humulin is
A)
antibiotic
done
clear
B)
insulin
done
clear
C)
haemoglobin
done
clear
D)
pepsin
done
clear
View Answer play_arrow