question_answer 1) Dimensions of which of the following three quantities are the same?
A)
Work, energy, force
done
clear
B)
Velocity, momentum, impulse
done
clear
C)
Potential energy, kinetic energy, momentum
done
clear
D)
Pressure, stress, coefficient of elasticity
done
clear
View Answer play_arrow
question_answer 2) From the following pairs, choose the pair that does not have identical dimensions.
A)
Angular momentum and Planck's constant
done
clear
B)
Moment of inertia and moment of force
done
clear
C)
Work and torque
done
clear
D)
Impulse and momentum
done
clear
View Answer play_arrow
question_answer 3) A body starts to fall freely under gravity. The distance covered by it in first, second and third second are in ratio
A)
1 : 3 : 5
done
clear
B)
1 : 2 : 3
done
clear
C)
1: 4: 9
done
clear
D)
1: 5 : 6
done
clear
View Answer play_arrow
question_answer 4) For a projectile on the horizontal surface of a planet \[y=8t-5{{t}^{2}}\] m shows height and \[x=8\,t\]m shows the horizontal distance, then the velocity with which the projectile is projected is
A)
8m/s
done
clear
B)
6 m/s
done
clear
C)
10 m/s
done
clear
D)
100 m/s
done
clear
View Answer play_arrow
question_answer 5) A motorcycle is going on an over bridge of radius R. The driver maintains a constant speed. As the motorcycle is ascending on the over bridge, the normal force on it
A)
increases
done
clear
B)
decreases
done
clear
C)
remains the same
done
clear
D)
fluctuates
done
clear
View Answer play_arrow
question_answer 6) A body of mass 0.5 kg is projected under gravity with a speed of 98 m/s at an angle of \[60{}^\circ \] with the horizontal. The change in momentum (in magnitude) of the body is
A)
24.5 N-s
done
clear
B)
49.0 N-s
done
clear
C)
98.0 N-s
done
clear
D)
50.0 N-s
done
clear
View Answer play_arrow
question_answer 7) A body of mass 10 kg is moving with a constant velocity of 10 m/s. When a constant force acts for 4 s on it, it moves with a velocity 2 m/s in the opposite direction. The acceleration produced in it is
A)
3 m/s2
done
clear
B)
- 3 m/s2
done
clear
C)
0.3 m/s2
done
clear
D)
- 0.3 m/s2
done
clear
View Answer play_arrow
question_answer 8) A body of mass M is kept on a rough horizontal surface [friction coefficient =µ). A person is trying to pull the body by applying a horizontal force F but the body is not moving then
A)
\[F=Mg\]
done
clear
B)
\[F=\mu Mg\]
done
clear
C)
\[Mg\le F\le Mg\sqrt{1+{{\mu }^{2}}}\]
done
clear
D)
\[Mg\ge F\ge Mg\sqrt{1+{{\mu }^{2}}}\]
done
clear
View Answer play_arrow
question_answer 9) A bullet of mass m moving with velocity v strikes a block of mass M at rest and gets embedded into it. The kinetic energy of the composite block will be
A)
\[\frac{1}{2}m{{v}^{2}}\times \frac{m}{(m+M)}\]
done
clear
B)
\[\frac{1}{2}m{{v}^{2}}\times \frac{M}{(m+M)}\]
done
clear
C)
\[\frac{1}{2}m{{v}^{2}}\times \frac{(M+m)}{M}\]
done
clear
D)
\[\frac{1}{2}M{{v}^{2}}\times \frac{m}{(m+M)}\]
done
clear
View Answer play_arrow
question_answer 10) One-fourth length of a spring of force constant k is cut away. The force constant of the remaining spring will be
A)
\[\frac{3}{4}k\]
done
clear
B)
\[\frac{4}{3}k\]
done
clear
C)
k
done
clear
D)
4k
done
clear
View Answer play_arrow
question_answer 11) The force constants of two springs are \[{{k}_{1}}\] and\[{{k}_{2}}\] respectively. Both are stretched till their potential energies are equal. The forces \[{{f}_{1}}\] and \[{{f}_{2}}\] applied on them are in the ratio
A)
\[{{k}_{1}}:{{k}_{2}}\]
done
clear
B)
\[{{k}_{2}}:{{k}_{1}}\]
done
clear
C)
\[\sqrt{{{k}_{1}}}:\sqrt{{{k}_{2}}}\]
done
clear
D)
\[\sqrt{{{k}_{2}}}:\sqrt{{{k}_{1}}}\]
done
clear
View Answer play_arrow
question_answer 12) A fighter plane is moving in a vertical circle of radius r. Its minimum velocity at the highest point of the circle will be
A)
\[\sqrt{3gr}\]
done
clear
B)
\[\sqrt{2gr}\]
done
clear
C)
\[\sqrt{gr}\]
done
clear
D)
\[\sqrt{gr/2}\]
done
clear
View Answer play_arrow
question_answer 13)
A spring of length L and uniform cross-section is spread on a smooth plane. One of its ends is pulled by a force F. Find the tension in it at a distance I from this end.
A)
\[\frac{l}{2}F\]
done
clear
B)
\[\frac{L}{l}F\]
done
clear
C)
\[\left( 1-\frac{l}{L} \right)F\]
done
clear
D)
\[\left( 1+\frac{l}{L} \right)F\]
done
clear
View Answer play_arrow
question_answer 14) A particle is making uniform circular motion with angular momentum J. If its kinetic energy is made half and angular frequency be doubled, its new angular momentum will be
A)
2J
done
clear
B)
4J
done
clear
C)
J/2
done
clear
D)
J/4
done
clear
View Answer play_arrow
question_answer 15)
Three rings each of mass M and radius R are arranged as shown in the figure. The moment of inertia of the system about YY' will be
A)
3 MR2
done
clear
B)
3/2 MR2
done
clear
C)
5 MR2
done
clear
D)
7/2 MR2
done
clear
View Answer play_arrow
question_answer 16) If the radius of the earth were to shrink by 1% its mass remaining the same, the acceleration due to gravity on the earth's surface would
A)
decrease by 1%
done
clear
B)
remain unchanged
done
clear
C)
increase by 1%
done
clear
D)
increase by 2%
done
clear
View Answer play_arrow
question_answer 17) A satellite is moving around the earth with speed v in a circular orbit of radius r. If the orbit radius is decreased by 1%, the speed of the satellite will
A)
increase by 1%
done
clear
B)
increase by 0.5%
done
clear
C)
decrease by 1%
done
clear
D)
decrease by 0.5%
done
clear
View Answer play_arrow
question_answer 18) How much energy will be necessary for making a body of 500 kg escape from the earth? \[\text{ }\!\![\!\!\text{ g}\,\text{=}\,\text{9}\text{.8}\,\text{m}{{\text{s}}^{2}},\] radius of the earth \[\text{6}\text{.4 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{6}}}\text{m }\!\!]\!\!\text{ }\]
A)
About \[9.8\times {{10}^{6}}J\]
done
clear
B)
About \[6.4\times {{10}^{8}}J\]
done
clear
C)
About \[3.1\times {{10}^{10}}J\]
done
clear
D)
About \[27.4\times {{10}^{12}}J\]
done
clear
View Answer play_arrow
question_answer 19) A wire of length L and cross-sectional area A is made of a material of Young's modulus Y. It is stretched by an amount x. The work done is
A)
\[YxA/2L\]
done
clear
B)
\[Y{{x}^{2}}A/L\]
done
clear
C)
\[Y{{x}^{2}}A/2L\]
done
clear
D)
\[2Y{{x}^{2}}A/L\]
done
clear
View Answer play_arrow
question_answer 20) A capillary tube, made of glass, is dipped into mercury. Then,
A)
mercury rises in the capillary tube
done
clear
B)
mercury rises and flows out of the capillary tube
done
clear
C)
mercury descends in the capillary tube
done
clear
D)
mercury neither rises nor descends in the capillary tube
done
clear
View Answer play_arrow
question_answer 21) At which of the following temperature the root mean square velocity of gaseous hydrogen molecules will be equal to root mean square velocity of oxygen molecules at \[47{}^\circ C\]. ?
A)
20 K
done
clear
B)
80 K
done
clear
C)
- 73 K
done
clear
D)
3 K
done
clear
View Answer play_arrow
question_answer 22) The pressure of a gas in a vessel is \[{{P}_{0.}}\] masses of all the molecules are halved and their speeds are doubled, the resulting pressure \[p\] will be
A)
\[4{{p}_{0}}\]
done
clear
B)
\[{{p}_{0}}/2\]
done
clear
C)
\[{{p}_{0}}\]
done
clear
D)
\[2{{p}_{0}}\]
done
clear
View Answer play_arrow
question_answer 23) For a gas, if\[\gamma =1.4,\] then atomicity \[{{C}_{p}}\] and\[{{C}_{v}}\] of the gas are respectively
A)
monoatomic, 5/2 R, 3/2 R
done
clear
B)
monoatomic, 7/2 R, 5/2 R
done
clear
C)
diatomic, 7/2 R, 5/2 R
done
clear
D)
triatomic, 7/2 R, 5/2 R
done
clear
View Answer play_arrow
question_answer 24) During the adiabatic expansion of 2 moles of a gas, the change in internal energy was found to be equal to -100 J. The work done during the process will be equal to
A)
zero
done
clear
B)
-100 J
done
clear
C)
200 J
done
clear
D)
100 J
done
clear
View Answer play_arrow
question_answer 25) In an adiabatic expansion of a gas initial and final temperatures are \[{{T}_{1}}\] and \[{{T}_{2}}\] respectively, then the change in internal energy of the gas is
A)
\[R\text{/ }\!\!\gamma\!\!\text{ }-1({{T}_{2}}-{{T}_{1}})\]
done
clear
B)
\[R\text{/ }\!\!\gamma\!\!\text{ }-1({{T}_{1}}-{{T}_{2}})\]
done
clear
C)
\[R({{T}_{2}}-{{T}_{1}})\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 26) The lengths and radii of two rods made of same material are in the ratio 1:2 and 2:3 respectively. If the temperature difference between the ends for the two rods be the same, then in the steady state, the amount of heat flowing per second through them will be in the ratio
A)
1 :1
done
clear
B)
4 : 3
done
clear
C)
8 : 9
done
clear
D)
3 : 2
done
clear
View Answer play_arrow
question_answer 27) Which of the following makes the best choice for cooking utensils?
A)
High specific heat and high conductivity
done
clear
B)
Low specific heat and low conductivity
done
clear
C)
High specific heat and low conductivity
done
clear
D)
Low specific heat and high conductivity
done
clear
View Answer play_arrow
question_answer 28) A hot liquid takes 5 min to cool from \[70{}^\circ C\] to \[60{}^\circ C\]. How long will it take to cool from \[60{}^\circ C\] to \[50{}^\circ C\]?
A)
5 min
done
clear
B)
more than 5 min
done
clear
C)
less than 5 min
done
clear
D)
less or more than 5 min depending on the nature of liquid
done
clear
View Answer play_arrow
question_answer 29) At temperature T, the power radiated by a body is Q watt. At the temperature 3T, the power radiated by it will be
A)
3Q
done
clear
B)
9Q
done
clear
C)
27 Q
done
clear
D)
81 Q
done
clear
View Answer play_arrow
question_answer 30) A particle of mass 10 g is executing simple harmonic motion with an amplitude of 0.5 m and periodic time of (\[\text{ }\!\!\pi\!\!\text{ /5}\]) second. The maximum value of the force acting on the particle is
A)
25 N
done
clear
B)
5 N
done
clear
C)
2.5 N
done
clear
D)
0.5 N
done
clear
View Answer play_arrow
question_answer 31) The period of vibration of a mass m suspended from a spring is 2 s. If along with it another mass of 2 kg is also suspended, the period of oscillation increases by one second. The mass m will be
A)
2 kg
done
clear
B)
1 kg
done
clear
C)
1.6kg
done
clear
D)
2.6 kg
done
clear
View Answer play_arrow
question_answer 32) A particle is executing simple harmonic motion with frequency\[f.\] The frequency at which its kinetic energy changes into potential energy is
A)
\[f\text{/2}\]
done
clear
B)
\[f\]
done
clear
C)
\[2f\]
done
clear
D)
\[4f\]
done
clear
View Answer play_arrow
question_answer 33) The lengths of two open organ pipes are \[l\] and \[(l+\Delta l)\] respectively. Neglecting end correction the frequency of beats between them will be approximately
A)
\[\frac{v}{2l}\]
done
clear
B)
\[\frac{v}{4l}\]
done
clear
C)
\[\frac{v\Delta l}{2{{l}^{2}}}\]
done
clear
D)
\[\frac{v\Delta l}{l}\] (here v is the speed of sound)
done
clear
View Answer play_arrow
question_answer 34) A source of sound is moving with constant velocity of 20 m/s emitting a note of frequency 1000 Hz. The ratio of frequencies observed by a stationary observer while the source is approaching him and after it crosses him will be (speed of sound v = 340 m/s)
A)
9 : 8
done
clear
B)
8 : 9
done
clear
C)
1:1
done
clear
D)
9 :10
done
clear
View Answer play_arrow
question_answer 35) The equation of a stationary wave is \[y=0.8\,\cos \,\left( \frac{\pi x}{20} \right)\,\sin \,200\,\pi t\] where x is in and t is in sec. The separation between consecutive nodes will be
A)
20 cm
done
clear
B)
10 cm
done
clear
C)
40cm
done
clear
D)
30cm
done
clear
View Answer play_arrow
question_answer 36) The distance travelled by light in glass (refractive index = 1.5) in a nanosecond will be
A)
45cm
done
clear
B)
40cm
done
clear
C)
30cm
done
clear
D)
20cm
done
clear
View Answer play_arrow
question_answer 37) Two phase related and monochromatic beams of light have intensities \[I\] and \[4I.\] Possible maximum and minimum intensities in the resultant beam obtained due to superposition are,
A)
\[5I\,\text{and}\,I\]
done
clear
B)
\[5I\,\text{and}\,3I\]
done
clear
C)
\[9I\,\text{and}\,3I\]
done
clear
D)
\[9I\,\text{and}\,I\]
done
clear
View Answer play_arrow
question_answer 38) A light ray is incident normally on a plane mirror. The angle of reflection will be
A)
\[135{}^\circ \]
done
clear
B)
\[90{}^\circ \]
done
clear
C)
\[45{}^\circ \]
done
clear
D)
\[0{}^\circ \]
done
clear
View Answer play_arrow
question_answer 39) If refractive indices of water and glass with respect to vacuum be 4/3 and 3/2 respectively, the refractive index of glass with respect to water will be
A)
9/8
done
clear
B)
8/9
done
clear
C)
1
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 40) Light passes from vacuum into glass at incident angle \[45{}^\circ \]. Which property of the light wave remains unchanged?
A)
Direction of motion
done
clear
B)
Speed
done
clear
C)
Wavelength
done
clear
D)
Frequency
done
clear
View Answer play_arrow
question_answer 41)
A convex lens has a focal length\[f.\] It is cut into two parts along the dotted line as shown in the figure. The focal length of each part will be
A)
\[f\text{/2}\]
done
clear
B)
\[f\]
done
clear
C)
\[\text{3/2}f\]
done
clear
D)
\[2f\]
done
clear
View Answer play_arrow
question_answer 42) The nature of sun's spectrum is
A)
continuous spectrum with absorption lines
done
clear
B)
lines spectrum
done
clear
C)
the spectrum of the helium atom
done
clear
D)
band spectrum
done
clear
View Answer play_arrow
question_answer 43) A defective eye cannot see close objects clearly because their image is formed
A)
on the eye lens
done
clear
B)
between eye lens and retina
done
clear
C)
on the retina
done
clear
D)
beyond retina
done
clear
View Answer play_arrow
question_answer 44) A telescope of diameter 2 m uses light of wavelength \[5000\,\,\overset{\text{o}}{\mathop{\text{A}}}\,\] for viewing stars. The minimum angular separation between two stars whose image is seen just resolved by this telescope is
A)
\[0.31\times {{10}^{-6}}\,\text{rad}\]
done
clear
B)
\[0.25\times {{10}^{-6}}\,\text{rad}\]
done
clear
C)
\[0.6\times {{10}^{-3}}\,\text{rad}\]
done
clear
D)
\[0.5\times {{10}^{-3}}\,\text{rad}\]
done
clear
View Answer play_arrow
question_answer 45) Velocity of light is minimum in
A)
vacuum
done
clear
B)
water
done
clear
C)
glass
done
clear
D)
diamond
done
clear
View Answer play_arrow
question_answer 46) A particle of mass m and charge q is accelerated through a potential difference of V volt. Its energy will be
A)
\[qV\]
done
clear
B)
\[mqV\]
done
clear
C)
\[(q/m)V\]
done
clear
D)
\[q/V\]
done
clear
View Answer play_arrow
question_answer 47) Photons of energy 6 eV are incident on a metal surface whose work function is 4 eV, The minimum kinetic energy of the emitted photoelectrons will be
A)
zero
done
clear
B)
1 eV
done
clear
C)
2eV
done
clear
D)
10 eV
done
clear
View Answer play_arrow
question_answer 48)
For the photoelectric effect, the maximum kinetic energy (KE) of the emitted photoelectrons is plotted against the frequency v of the incident photons as shown in the figure. The slope of the curve gives
A)
charge of the electron
done
clear
B)
work function of the metal
done
clear
C)
Planck's constant
done
clear
D)
ratio of the Planck's constant to electronic charge
done
clear
View Answer play_arrow
question_answer 49) The unit of Planck's constant h is that of
A)
energy
done
clear
B)
work
done
clear
C)
linear momentum
done
clear
D)
angular momentum
done
clear
View Answer play_arrow
question_answer 50) Light of frequency \[4{{v}_{0}}\] is incident on the metal of the threshold frequency \[{{v}_{0}}.\] The maximum kinetic energy of the emitted photoelectrons is
A)
\[3h{{v}_{0}}\]
done
clear
B)
\[2h{{v}_{0}}\]
done
clear
C)
\[\frac{3}{2}h{{v}_{0}}\]
done
clear
D)
\[\frac{1}{2}h{{v}_{0}}\]
done
clear
View Answer play_arrow
question_answer 51) An electron and a proton are kept in a uniform electric field. The ratio of their acceleration will be
A)
unity
done
clear
B)
zero
done
clear
C)
\[{{\text{m}}_{p}}\text{/}{{\text{m}}_{\text{e}}}\]
done
clear
D)
\[{{\text{m}}_{e}}\text{/}{{\text{m}}_{p}}\]
done
clear
View Answer play_arrow
question_answer 52) In a X-ray tube. X-rays are produced by electrons accelerated by V volt. The maximum frequency of the X-rays is
A)
\[ehV\]
done
clear
B)
\[hV\text{/e}\]
done
clear
C)
\[eh\text{/V}\]
done
clear
D)
\[eV\text{/h}\]
done
clear
View Answer play_arrow
question_answer 53) The penetrating power of X-rays can be increased by
A)
increasing the current in the filament
done
clear
B)
decreasing the current in the filament
done
clear
C)
increasing the potential difference between the cathode and the anode
done
clear
D)
decreasing the potential difference between the cathode and the anode
done
clear
View Answer play_arrow
question_answer 54) Two wires P and Q made up of different materials have same resistance at room temperature, when heated, resistance of P increases and that of Q decreases. We conclude that
A)
P and Q both are conductors but because of being made of different materials it happens so
done
clear
B)
P is n-type semiconductor and Q is p-type semiconductor
done
clear
C)
P is semiconductor and Q is conductor
done
clear
D)
P is conductor and Q is semiconductor
done
clear
View Answer play_arrow
question_answer 55) The band gap in germanium and silicon in eV respectively is
A)
0.7,1.1
done
clear
B)
1.1, 0.7
done
clear
C)
1.1, 0
done
clear
D)
0,1.1
done
clear
View Answer play_arrow
question_answer 56)
A full wave rectifier circuit along with the input and output voltages is shown in the figure.
A)
A, C
done
clear
B)
B, D
done
clear
C)
B,C
done
clear
D)
A,D
done
clear
View Answer play_arrow
question_answer 57) The electron in the lowest (n = 1) orbit in hydrogen atom has energy - 13.6 eV. How much energy is required to ionize a hydrogen atom which is already in the first excited level?
A)
3.4 eV
done
clear
B)
10.2 eV
done
clear
C)
13.6 eV
done
clear
D)
1.9 eV
done
clear
View Answer play_arrow
question_answer 58) The ratio of minimum to maximum wavelength in Balmer series is
A)
5 : 9
done
clear
B)
5 : 36
done
clear
C)
1 : 4
done
clear
D)
3 : 4
done
clear
View Answer play_arrow
question_answer 59) A nucleus \[_{z}^{A}X\] emits an a-particle. The resultant nucleus emits \[a\text{ }\!\!\beta\!\!\text{ -}\]particle. The respective atomic and mass numbers of the final nucleus will be
A)
Z - 3, A - 4
done
clear
B)
Z -1, A - 4
done
clear
C)
Z - 2, A - 4
done
clear
D)
Z, A - 2
done
clear
View Answer play_arrow
question_answer 60) What fraction of a radioactive material will get disintegrated in a period of two half-lives?
A)
Whole
done
clear
B)
Half
done
clear
C)
One-fourth
done
clear
D)
Three-fourth
done
clear
View Answer play_arrow
question_answer 61) At 0 K, intrinsic semiconductors behaves as
A)
a perfect conductor
done
clear
B)
a super conductor
done
clear
C)
a semiconductor
done
clear
D)
a perfect insulator
done
clear
View Answer play_arrow
question_answer 62) To obtain electrons as majority charge carries in a semiconductor, the impurity mixed is
A)
monovalent
done
clear
B)
divalent
done
clear
C)
trivalent
done
clear
D)
pentavalent
done
clear
View Answer play_arrow
question_answer 63) The ratio of forward biased to reverse biased resistance for p-n junction diode is
A)
10-1 :1
done
clear
B)
10-2:1
done
clear
C)
10-3 :1
done
clear
D)
10-2 : 1
done
clear
View Answer play_arrow
question_answer 64) For germanium crystal, the forbidden energy gap in joule is
A)
\[1.12\times {{10}^{-19}}\]
done
clear
B)
\[1.76\times {{10}^{-19}}\]
done
clear
C)
\[1.6\times {{10}^{-19}}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 65) The incorrect statement regarding the lines of force of the magnetic field B is
A)
magnetic intensity is a measure of lines of force passing through unit area held normal to it
done
clear
B)
magnetic lines of force form a close curve
done
clear
C)
inside a magnet, its magnetic lines of force move from north pole of a magnet towards its south pole
done
clear
D)
magnetic lines of force never cut each other
done
clear
View Answer play_arrow
question_answer 66) A bar magnet of magnetic moment M and length L is cut into two equal parts each of length L/2. The magnetic moment of each part will be
A)
M
done
clear
B)
M/4
done
clear
C)
\[\sqrt{2M}\]
done
clear
D)
M/2
done
clear
View Answer play_arrow
question_answer 67) When 2 A current is passed through a tangent galvanometer, it gives a deflection of \[30{}^\circ \]. For \[60{}^\circ \] deflection, the current must be
A)
1A
done
clear
B)
\[2\sqrt{3}\text{A}\]
done
clear
C)
4A
done
clear
D)
6 A
done
clear
View Answer play_arrow
question_answer 68) At a certain place the horizontal component of the earth's magnetic field is \[{{B}_{0}}\] and the angle of dip is \[45{}^\circ \]. The total intensity of the field at that place will be
A)
\[{{B}_{0}}\]
done
clear
B)
\[\sqrt{2}{{B}_{0}}\]
done
clear
C)
\[2B_{0}^{{}}\]
done
clear
D)
\[B_{0}^{2}\]
done
clear
View Answer play_arrow
question_answer 69) The field at a point distant r from an electric dipole is proportional to
A)
1/r
done
clear
B)
1/r2
done
clear
C)
1/r3
done
clear
D)
r2
done
clear
View Answer play_arrow
question_answer 70) What is the magnitude of a point charge which produces an electric field of 2 N/C at a distance of 60 cm? \[[\frac{1}{4\pi {{\varepsilon }_{0}}}=9\times {{10}^{9}}\text{N-}{{\text{m}}^{\text{2}}}\text{/}{{\text{C}}^{\text{2}}}\text{ }\!\!]\!\!\text{ }\]
A)
\[8\times {{10}^{11}}C\]
done
clear
B)
\[2\times {{10}^{-12}}C\]
done
clear
C)
\[3\times {{10}^{-11}}C\]
done
clear
D)
\[6\times {{10}^{-10}}C\]
done
clear
View Answer play_arrow
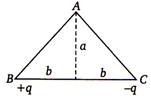
question_answer 71)
As shown in the figure, charges +q and - q are placed at the vertices B and C of an isosceles triangle. The potential at the vertex A is
A)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}.\frac{2q}{\sqrt{{{a}^{2}}+{{b}^{2}}}}\]
done
clear
B)
\[\text{zero}\]
done
clear
C)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}.\frac{q}{\sqrt{{{a}^{2}}+{{b}^{2}}}}\]
done
clear
D)
\[\frac{1}{4\pi {{\varepsilon }_{0}}}.\frac{(-q)}{\sqrt{{{a}^{2}}+{{b}^{2}}}}\]
done
clear
View Answer play_arrow
question_answer 72) A hollow metal sphere of radius 5 cm is charged such that the potential on its surface is 10 V. The electric field at the centre of the sphere will be
A)
50V/m
done
clear
B)
10V/m
done
clear
C)
5V/m
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 73) Angle between equipotential surface and lines of force is
A)
zero
done
clear
B)
\[180{}^\circ \]
done
clear
C)
\[90{}^\circ \]
done
clear
D)
\[45{}^\circ \]
done
clear
View Answer play_arrow
question_answer 74) After fully charging a parallel plate air capacitor, battery is removed. A dielectric slab is now put between the plates. For this incidence, the correct statement is
A)
charge on plates decreases
done
clear
B)
charge on plates does not change , potential difference increases
done
clear
C)
charge on plates does not change? potential difference decreases and energy stored decreases
done
clear
D)
charge on plates does not change, potential difference increases and energy stored also increases
done
clear
View Answer play_arrow
question_answer 75) A hollow metallic sphere of radius 3 cm 5 charged so that potential on its surface becomes 5 V. The potential at the centre of sphere will be (in volt)
A)
zero
done
clear
B)
5
done
clear
C)
3
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 76) When a potential is applied across the ends of a linear metallic conductor, then
A)
the free electrons are accelerated continuously from the lower potential end to the higher potential end of the conductor
done
clear
B)
the free electron are accelerated continuously from the higher potential end to the lower potential end of the conductor
done
clear
C)
the free electrons acquire a constant drift velocity from the lower potential end to the higher potential end of the conductor
done
clear
D)
the free electrons are set in motion from their position of rest
done
clear
View Answer play_arrow
question_answer 77) In a neon discharge tube\[2.9\times {{10}^{18}}\] Ne ions move to the right each second, while\[1.2\times {{10}^{18}}\] electrons move to the left per sec, electron charge is \[1.6\times {{10}^{-19}}C.\] The current in the discharge tube is
A)
1 A towards right
done
clear
B)
0.66 A towards right
done
clear
C)
0.66 A towards left
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 78) When a resistance of 2\[\Omega \] is connected across the terminals of a cell, the current is 0.5 A. When the resistance is increased to 5\[\Omega ,\] the current is 0.25 A. The emf of the cell is
A)
1.0 V
done
clear
B)
1.5V
done
clear
C)
2.0V
done
clear
D)
2.5V
done
clear
View Answer play_arrow
question_answer 79) An electric heater, kept in vacuum, is heated continuously by passing electric current. Its temperature
A)
will go on rising with time
done
clear
B)
will stop rising after sometime as it will lose heat to the surroundings by conduction
done
clear
C)
will rise for sometime and thereafter will start falling
done
clear
D)
will become constant after sometime because of loss of heat due to radiation
done
clear
View Answer play_arrow
question_answer 80) Heat produced in a wire of resistance R due to current flowing at a constant potential difference is proportional to
A)
1/R2
done
clear
B)
1/R
done
clear
C)
R
done
clear
D)
R2
done
clear
View Answer play_arrow
question_answer 81) There are two electric bulbs of 40 W and 100 W. Which one will be brighter when first they are connected in series and then in parallel
A)
40 W in series and 100 W in parallel
done
clear
B)
100 W in series and 40 W in parallel
done
clear
C)
40 W both in series and parallel will be uniform
done
clear
D)
100 W both in series and parallel will be uniform
done
clear
View Answer play_arrow
question_answer 82) In charging a battery of motor car, the following effect of electric current is used
A)
magnetic
done
clear
B)
heating
done
clear
C)
chemical
done
clear
D)
induction
done
clear
View Answer play_arrow
question_answer 83) To convert the range of a G ohm resistance voltmeter from V to nV, the value of series resistance needed is
A)
\[(n-1)G\]
done
clear
B)
\[\text{G/}n\]
done
clear
C)
\[nG\]
done
clear
D)
\[\text{G/}(n-1)\]
done
clear
View Answer play_arrow
question_answer 84) If the angular momentum of an electron is \[\mathbf{\vec{J}},\] then the magnitude of the magnetic moment will be
A)
\[\frac{eJ}{m}\]
done
clear
B)
\[\frac{eJ}{2m}\]
done
clear
C)
\[eJ2m\]
done
clear
D)
\[\frac{2m}{eJ}\]
done
clear
View Answer play_arrow
question_answer 85) A 100\[\Omega \] galvanometer gives full scale deflection at 10 mA. How much shunt is required to read 100 mA?
A)
\[11.11\,\Omega \]
done
clear
B)
\[9.9\,\Omega \]
done
clear
C)
\[1.1\,\Omega \]
done
clear
D)
\[4.4\,\Omega \]
done
clear
View Answer play_arrow
question_answer 86) The ratio of the magnetic field at the centre of a current carrying coil of radius a and at a distance x from centre of the coil on its axial line is a
A)
\[\frac{1}{\sqrt{2}}\]
done
clear
B)
\[\sqrt{2}\]
done
clear
C)
\[\frac{1}{2\sqrt{2}}\]
done
clear
D)
\[2\sqrt{2}\]
done
clear
View Answer play_arrow
question_answer 87) On connecting a battery to the two comers of a diagonal of a square conductor frame of side \[a,\] the magnitude of the magnetic field at the centre will be
A)
zero
done
clear
B)
\[\frac{{{\mu }_{0}}}{\pi a}\]
done
clear
C)
\[\frac{2{{\mu }_{0}}}{\pi a}\]
done
clear
D)
\[\frac{4{{\mu }_{0}}i}{\pi a}\]
done
clear
View Answer play_arrow
question_answer 88) Lorentz force is given by the following formula
A)
\[\mathbf{\vec{F}}=q\mathbf{\vec{E}}\]
done
clear
B)
\[\mathbf{\vec{F}}=q(\mathbf{\vec{V}}\times \mathbf{\vec{B}})\]
done
clear
C)
\[\mathbf{\vec{F}}=q\mathbf{\vec{E}}+q(\mathbf{\vec{V}}\times \mathbf{\vec{B}})\]
done
clear
D)
\[\mathbf{\vec{F}}=q\mathbf{\vec{E}}+q(\mathbf{\vec{B}}\times \mathbf{\vec{V}})\]
done
clear
View Answer play_arrow
question_answer 89) When a charged particle enters in a uniform magnetic field, its kinetic energy
A)
remains constant
done
clear
B)
increases
done
clear
C)
decreases
done
clear
D)
becomes zero
done
clear
View Answer play_arrow
question_answer 90) A long sol enoid has n turns per metre and current \[I\] ampere is flowing through it. The magnetic field at the ends of solenoid is
A)
\[\frac{{{\mu }_{0}}nI}{2}\]
done
clear
B)
\[{{\mu }_{0}}nI\]
done
clear
C)
zero
done
clear
D)
\[2{{\mu }_{0}}nI\]
done
clear
View Answer play_arrow
question_answer 91) Biot-Savart law is given by the following formula [here k=\[({{\mu }_{0}}\text{/4 }\!\!\pi\!\!\text{ })]\]
A)
\[d\,\mathbf{\vec{B}}=ki\frac{d\mathbf{\vec{l}}\times \mathbf{\hat{r}}}{{{r}^{3}}}\]
done
clear
B)
\[d\,\mathbf{\vec{B}}=k{{i}^{2}}\frac{d\mathbf{\vec{l}}\times \mathbf{\hat{r}}}{{{r}^{3}}}\]
done
clear
C)
\[d\,\mathbf{\vec{B}}=ki\frac{d\mathbf{\vec{l}}\times \mathbf{\vec{r}}}{{{r}^{3}}}\]
done
clear
D)
\[d\,\mathbf{\vec{B}}=ki\frac{\mathbf{\vec{r}}\times d\,\mathbf{\vec{l}}}{{{r}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 92) A circular coil of 500 turns of wire has an enclosed area of 0.1 m2 per turn. It is kept perpendicular to a magnetic field of induction 0.2 T and rotated by \[180{}^\circ \] about a diameter perpendicular to the field in 0.1 s. How much charge will pass when the coil is connected to a galvanometer with a combined resistance of son?
A)
0.2 C
done
clear
B)
0.4 C
done
clear
C)
2 C
done
clear
D)
4 C
done
clear
View Answer play_arrow
question_answer 93) The self-inductance of a solenoid of length \[l,\] area of cross-section A and having N turns is
A)
\[{{\mu }_{0}}{{N}^{2}}\text{A/}l\]
done
clear
B)
\[{{\mu }_{0}}N\text{A/}l\]
done
clear
C)
\[{{\mu }_{0}}{{N}^{2}}l\text{A}\]
done
clear
D)
\[{{\mu }_{0}}N\text{A}l\]
done
clear
View Answer play_arrow
question_answer 94) The frequency for which a 5µF capacitor has a reactance of 1/1000\[\Omega \] is given by
A)
100/\[\pi \]MHz
done
clear
B)
1000/\[\pi \]Hz
done
clear
C)
1/1000 Hz
done
clear
D)
1000 Hz
done
clear
View Answer play_arrow
question_answer 95) The unit of inductance is
A)
Volt/Ampere
done
clear
B)
Joule/Ampere
done
clear
C)
Volt x Second/Ampere
done
clear
D)
Volt x Ampere/Second
done
clear
View Answer play_arrow
question_answer 96) An alternating emf is applied to purely capacitive circuit. The phase relation between emf and current flowing in the circuit is
A)
emf is ahead of current by \[\text{ }\!\!\pi\!\!\text{ /2}\]
done
clear
B)
current is ahead of emf by\[\text{ }\!\!\pi\!\!\text{ /2}\]
done
clear
C)
current lags behind emf by\[\text{ }\!\!\pi\!\!\text{ }\]
done
clear
D)
current is ahead of emf by \[\text{ }\!\!\pi\!\!\text{ }\]
done
clear
View Answer play_arrow
question_answer 97) In a circuit, the value of the alternating current is measured by an ammeter as 10 A. Its peak value will be
A)
10 A
done
clear
B)
20 A
done
clear
C)
14.14 A
done
clear
D)
7.07 A
done
clear
View Answer play_arrow
question_answer 98) In a series L-C-R circuit, operated with an AC are angular frequency a, the total impedance s
A)
\[{{[{{R}^{2}}+{{(L\omega -C\omega )}^{2}}]}^{1/2}}\]
done
clear
B)
\[{{\left[ {{R}^{2}}+{{\left( L\omega -\frac{1}{C\omega } \right)}^{2}} \right]}^{1/2}}\]
done
clear
C)
\[{{\left[ {{R}^{2}}+{{\left( L\omega -\frac{1}{C\omega } \right)}^{2}} \right]}^{1/2}}\]
done
clear
D)
\[{{\left[ {{(R\omega )}^{2}}+{{\left( L\omega -\frac{1}{C\omega } \right)}^{2}} \right]}^{1/2}}\]
done
clear
View Answer play_arrow
question_answer 99) In a L-R circuit, the value of L is \[(0.\text{4/ }\!\!\pi\!\!\text{ })\] H sar the value of R is 30\[\Omega \]. If in circuit, an alternating emf of 200 V at 50 cycle/s is connected, the impedance of the circuit and current will be
A)
11.\[\Omega ,\] 17.5A
done
clear
B)
30.\[\Omega ,\]6.5A
done
clear
C)
40.\[\Omega ,\] 5 A
done
clear
D)
50\[\Omega ,\] 4 A
done
clear
View Answer play_arrow
question_answer 100) The coils of a step down transformer have 501 and 5000 turns. In the primary coil an AC of 4 A at 2200 V is sent. The value of the current and potential difference in secondary coil will be
A)
20 A, 220V
done
clear
B)
0.4 A, 22000V
done
clear
C)
40 A, 220 V
done
clear
D)
40 A, 22000 V
done
clear
View Answer play_arrow
question_answer 101) The maximum number of electrons in an atom with \[l=2\] and \[n=3\] is
A)
\[2\]
done
clear
B)
\[6\]
done
clear
C)
\[12\]
done
clear
D)
\[10\]
done
clear
View Answer play_arrow
question_answer 102) Which of the following expressions gives the de-Broglie relationship?
A)
\[\frac{h}{mv}=p\]
done
clear
B)
\[\lambda =\frac{h}{mv}\]
done
clear
C)
\[\lambda =\frac{h}{mp}\]
done
clear
D)
\[\lambda m=\frac{v}{p}\]
done
clear
View Answer play_arrow
question_answer 103) What is the electronic configuration: \[C{{u}^{2+}}(Z=29)\] of least position?
A)
\[[Ar]4{{s}^{1}},3{{d}^{8}}\]
done
clear
B)
\[[Ar]4{{s}^{2}},3{{d}^{10}},4{{p}^{1}}\]
done
clear
C)
\[[Ar]4{{s}^{1}},3{{d}^{10}}\]
done
clear
D)
\[[Ar]3{{d}^{9}}\]
done
clear
View Answer play_arrow
question_answer 104) Which of the following orbitals has a dumb-bell shape?
A)
\[s\]
done
clear
B)
\[p\]
done
clear
C)
\[d\]
done
clear
D)
\[f\]
done
clear
View Answer play_arrow
question_answer 105) The electronic configuration of metal M is \[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{1}},\]The formula of its oxide will be
A)
\[MO\]
done
clear
B)
\[{{M}_{2}}O\]
done
clear
C)
\[{{M}_{2}}{{O}_{3}}\]
done
clear
D)
\[M{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 106) Which of the following statements regarding covalent bond is not true?
A)
The electrons are shared between atoms.
done
clear
B)
The bond is non-directional.
done
clear
C)
The strength of the bond depends upon the extent of overlapping.
done
clear
D)
The bonds formed may or may not be polar.
done
clear
View Answer play_arrow
question_answer 107) Directed bonds in water form an angle of
A)
\[{{90}^{o}}\]
done
clear
B)
\[{{120}^{o}}\]
done
clear
C)
\[{{104.5}^{o}}\]
done
clear
D)
\[{{60}^{o}}\]
done
clear
View Answer play_arrow
question_answer 108) Hydrogen bonding is not present in
A)
glycerine
done
clear
B)
water
done
clear
C)
hydrogen sulphide
done
clear
D)
hydrogen fluoride
done
clear
View Answer play_arrow
question_answer 109) \[s{{p}^{3}}\]hybridisation leads to which shape of the molecule?
A)
Tetrahedron
done
clear
B)
Octahedron
done
clear
C)
Linear
done
clear
D)
Plane triangle
done
clear
View Answer play_arrow
question_answer 110) Which one of the following is not an ideal solution?
A)
\[{{C}_{2}}{{H}_{5}}Br,{{C}_{2}}{{H}_{5}}I\,\,mixture\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}Cl,{{C}_{6}}{{H}_{5}}Br\,mixture\]
done
clear
C)
\[{{C}_{6}}{{H}_{6}},{{C}_{6}}{{H}_{5}}C{{H}_{3}}\,mixture\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}I,{{C}_{2}}{{H}_{5}}OH\,mixture\]
done
clear
View Answer play_arrow
question_answer 111) "The relative lowering of vapour pressure is equal to the mole fraction of the solute." This law is called
A)
Henry's law
done
clear
B)
Raoult's law
done
clear
C)
Ostwald's law
done
clear
D)
Arrhenius's law
done
clear
View Answer play_arrow
question_answer 112) The order of osmotic pressures of equimolar solutions of \[BaC{{l}_{2}},\]\[~NaCl\] and glucose will be
A)
\[BaC{{l}_{2}}>NaCl>glucose\]
done
clear
B)
\[NaCl>BaC{{l}_{2}}>glucose\]
done
clear
C)
\[glucose>BaC{{l}_{2}}>NaCl\]
done
clear
D)
\[glucose>NaCl>BaC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 113) Molal depression constant for water is\[{{1.86}^{o}}C\] The freezing point of a \[0.05\]molal solution of a non-electrolyte in water, is
A)
\[-{{1.86}^{o}}C\]
done
clear
B)
\[-{{0.93}^{o}}C\]
done
clear
C)
\[-{{0.093}^{o}}C~\]
done
clear
D)
\[{{0.93}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 114) How many mL of \[1M\] \[{{H}_{2}}S{{O}_{4}}\] is required to neutralise \[10\text{ }mL\]of \[1M\] \[NaOH\]solution?
A)
\[2.5\]
done
clear
B)
\[5.0\]
done
clear
C)
\[10.0\]
done
clear
D)
\[20.0\]
done
clear
View Answer play_arrow
question_answer 115) The correct statement in the following is
A)
The ionic crystal of \[AgBr\]has Schottky defect
done
clear
B)
The unit cell having crystal parameters, \[a=b\ne c,\] \[\alpha =\beta ={{90}^{o}},\] \[\gamma ={{120}^{o}}\]is hexagonal
done
clear
C)
In ionic compounds having Frenkel defect, the ratio \[{{r}_{+}}/{{r}_{-}}\] is high
done
clear
D)
The coordination number of \[N{{a}^{+}}\] ion in \[NaCl\]is 4
done
clear
View Answer play_arrow
question_answer 116) The number of atoms in unit cell of \[NaCl\]crystal is
A)
\[2\]
done
clear
B)
\[4\]
done
clear
C)
\[6\]
done
clear
D)
\[8\]
done
clear
View Answer play_arrow
question_answer 117) The average life period of a radioactive element is reciprocal of its
A)
half-life period
done
clear
B)
disintegration constant
done
clear
C)
number of atoms present at any time
done
clear
D)
number of neutrons
done
clear
View Answer play_arrow
question_answer 118) Uranium ultimately decays into a stable isotope of
A)
radium
done
clear
B)
carbon
done
clear
C)
lead
done
clear
D)
neptunium
done
clear
View Answer play_arrow
question_answer 119) An isotope \[_{Y}^{X}A\] undergoes a series of m alpha and n beta disintegration to form a stable isotope\[_{Y-10}^{X-32}B\]. The values of m and n are respectively
A)
\[6\] and \[8\]
done
clear
B)
\[8\] and \[10\]
done
clear
C)
\[5\] and \[8\]
done
clear
D)
\[8\] and \[6\]
done
clear
View Answer play_arrow
question_answer 120) A substance used as a moderator in a nuclear reactor is
A)
cadmium
done
clear
B)
uranium-235
done
clear
C)
lead
done
clear
D)
heavy water
done
clear
View Answer play_arrow
question_answer 121) For which one of the following reactions\[{{K}_{p}}={{K}_{c}}\]?
A)
\[{{N}_{2}}+3{{H}_{2}}\rightleftharpoons 2N{{H}_{3}}\]
done
clear
B)
\[{{N}_{2}}+{{O}_{2}}\rightleftharpoons 2NO\]
done
clear
C)
\[PC{{l}_{5}}\rightleftharpoons PC{{l}_{3}}+C{{l}_{2}}\]
done
clear
D)
\[2S{{O}_{3}}\rightleftharpoons 2S{{O}_{2}}+{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 122) The equilibrium constant for the reversible reaction, \[{{N}_{2}}+3{{H}_{2}}\rightleftharpoons 2N{{H}_{3}}\] is K and for the reaction \[\frac{1}{2}{{N}_{2}}+\frac{3}{2}{{H}_{2}}\rightleftharpoons N{{H}_{3}},\] the equilibrium constant is \[K'\]. K and \[K'\] will be related as
A)
\[K=K'\]
done
clear
B)
\[K=\sqrt{K}\]
done
clear
C)
\[K=\sqrt{K'}\]
done
clear
D)
\[K\times K'=1\]
done
clear
View Answer play_arrow
question_answer 123) At \[{{20}^{o}}C\]the \[A{{g}^{+}}\] ion concentration in a saturated solution of \[A{{g}_{2}}Cr{{O}_{4}}\] is \[1.5\times {{10}^{-4}}mol/L\]. At \[{{20}^{o}}C\]the solubility product of \[A{{g}_{2}}Cr{{O}_{4}}\] would be
A)
\[3.3750\times {{10}^{-12}}~\]
done
clear
B)
\[1.6875\times {{10}^{-10}}\]
done
clear
C)
\[1.6875\times {{10}^{-12}}\]
done
clear
D)
\[1.6875\times {{10}^{-11}}\]
done
clear
View Answer play_arrow
question_answer 124) What will be the pH of a \[{{10}^{-8}}M\]\[HCl\] solution?
A)
\[8.0\]
done
clear
B)
\[7.0\]
done
clear
C)
\[6.98\]
done
clear
D)
\[14.0\]
done
clear
View Answer play_arrow
question_answer 125) Which of the following can act both as Bronsted acid and a Bronsted base?
A)
\[N{{a}_{2}}C{{O}_{3}}\]
done
clear
B)
\[O{{H}^{-}}\]
done
clear
C)
\[HC{{O}_{3}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 126) What will be the sum of pH and pOH in an aqueous solution?
A)
\[7\]
done
clear
B)
\[p{{K}_{w}}\]
done
clear
C)
Zero
done
clear
D)
\[1\]
done
clear
View Answer play_arrow
question_answer 127) Given that the dissociation constant for \[{{H}_{2}}O\] is \[{{K}_{w}}=1\times {{10}^{-14}}\] what is the pH of a M \[KOH\] solution?
A)
\[11\]
done
clear
B)
\[3\]
done
clear
C)
\[14\]
done
clear
D)
\[{{10}^{-11}}\]
done
clear
View Answer play_arrow
question_answer 128) The relation between \[\Delta G\] and E for cell is \[\Delta G=-nFE;\] the cell reaction will be spontaneous, if
A)
G is negative
done
clear
B)
G is positive
done
clear
C)
E is negative
done
clear
D)
E is positive
done
clear
View Answer play_arrow
question_answer 129) For an adiabatic process
A)
\[Q=0\]
done
clear
B)
\[Q=-W\]
done
clear
C)
\[Q=+W\]
done
clear
D)
\[p\,\Delta V=0\]where Q, W, p and AV denote heat, work, pressure and change in volume respectively.
done
clear
View Answer play_arrow
question_answer 130) Decrease of free energy of a reacting system points to a/an
A)
exothermic reaction
done
clear
B)
equilibrium reaction
done
clear
C)
spontaneous reaction
done
clear
D)
isothermal reaction
done
clear
View Answer play_arrow
question_answer 131) When a solid is converted into liquid, entropy
A)
becomes zero
done
clear
B)
remains the same
done
clear
C)
decreases
done
clear
D)
increases
done
clear
View Answer play_arrow
question_answer 132) Carbon and carbon monoxide bum in oxygen to form carbon dioxide according to the following reactions \[C+{{O}_{2}}\xrightarrow{{}}C{{O}_{2}};\] \[\Delta H=-394\,kJ\,mo{{l}^{-1}}\] \[2CO+{{O}_{2}}\xrightarrow{{}}2C{{O}_{2}};\] \[\Delta H=-569\,kJ\,mo{{l}^{-1}}\] The heat of formation of \[1\text{ }mole\]of monoxide is, thus
A)
\[-219.0\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
B)
\[-109.5\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
C)
\[-175.0\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
D)
\[-87.5\text{ }kJ\text{ }mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 133) The thermal decomposition of a compound is of first order. If a sample of the compound decomposes 50% in 120 min, in what time will it undergo 90% decomposition?
A)
\[Nearly\text{ }240\text{ }min\]
done
clear
B)
\[Nearly\text{ }480\text{ }min\]
done
clear
C)
\[Nearly\text{ }450\text{ }min\]
done
clear
D)
\[Nearly\text{ }400\text{ }min\]
done
clear
View Answer play_arrow
question_answer 134) The order of a reaction with rate equal to \[kC_{A}^{3/2}C_{B}^{-1/2},\] is
A)
\[2\]
done
clear
B)
\[1\]
done
clear
C)
\[-\frac{1}{2}\]
done
clear
D)
\[\frac{3}{2}\]
done
clear
View Answer play_arrow
question_answer 135) The unit of cell constant is
A)
\[oh{{m}^{-1}}\,c{{m}^{-1}}\]
done
clear
B)
\[ohm\text{ }cm\]
done
clear
C)
\[cm~\]
done
clear
D)
\[cm{{~}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 136) The chemical reaction taking place at he anode of a cell is
A)
ionisation
done
clear
B)
reduction
done
clear
C)
oxidation
done
clear
D)
hydrolysis
done
clear
View Answer play_arrow
question_answer 137) In dry cell, the reaction which takes place at the graphite anode is
A)
\[Z{{n}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Zn(s)\]
done
clear
B)
\[Zn(s)+2{{e}^{-}}\xrightarrow{{}}Zn(s)\]
done
clear
C)
\[M{{n}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Mn(s)\]
done
clear
D)
\[Mn(s)\xrightarrow{{}}M{{n}^{+}}+{{e}^{-}}1.5V\]
done
clear
View Answer play_arrow
question_answer 138) The metal which gives hydrogen on treatment with acid as well as sodium hydroxide is
A)
iron
done
clear
B)
zinc
done
clear
C)
copper
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 139) Chlorine cannot displace
A)
fluorine from \[NaF\]
done
clear
B)
iodine from \[NaI\]
done
clear
C)
bromine from \[NaBr\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 140) Milk is an example of
A)
emulsion
done
clear
B)
suspension
done
clear
C)
sol
done
clear
D)
gel
done
clear
View Answer play_arrow
question_answer 141) Fog is a colloidal system of
A)
gas in liquid
done
clear
B)
liquid in gas
done
clear
C)
gas in gas
done
clear
D)
solid in gas
done
clear
View Answer play_arrow
question_answer 142) In physical adsorption, the gas molecules are held on solid surface by
A)
chemical forces
done
clear
B)
electrostatic forces
done
clear
C)
gravitational forces
done
clear
D)
van der Waals' forces
done
clear
View Answer play_arrow
question_answer 143) Hoope's process is used in the refining of
A)
\[Al\]
done
clear
B)
\[Zn\]
done
clear
C)
\[Ag\]
done
clear
D)
\[Cu\]
done
clear
View Answer play_arrow
question_answer 144) Red lead is
A)
\[P{{b}_{3}}{{O}_{4}}\]
done
clear
B)
\[PbO\]
done
clear
C)
\[Pb{{O}_{2}}\]
done
clear
D)
\[P{{b}_{4}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 145) Solder metal is
A)
\[Pb\text{ + }Sb\]
done
clear
B)
\[Pb+Sn\]
done
clear
C)
\[Cu+Sn\]
done
clear
D)
\[Cu+Zn\]
done
clear
View Answer play_arrow
question_answer 146) In the manufacture of iron from hematite the function of lime stone is as
A)
reducing agent
done
clear
B)
flux
done
clear
C)
slag
done
clear
D)
gangue
done
clear
View Answer play_arrow
question_answer 147) Blister copper is
A)
pure copper
done
clear
B)
copper ore
done
clear
C)
2% impure copper
done
clear
D)
copper alloy
done
clear
View Answer play_arrow
question_answer 148) The purest from of iron is
A)
cast iron
done
clear
B)
pig iron
done
clear
C)
wrought iron
done
clear
D)
steel
done
clear
View Answer play_arrow
question_answer 149) Who developed the long form of periodic table?
A)
Lothar Meyer
done
clear
B)
Neils Bohr
done
clear
C)
Mendeleef
done
clear
D)
Moseley
done
clear
View Answer play_arrow
question_answer 150) The correct sequence of elements in decreasing order of first ionisation energy is
A)
\[Na>Mg>Al~\]
done
clear
B)
\[Mg>Na>Al\]
done
clear
C)
\[Al>Mg>Na~\]
done
clear
D)
\[Mg>Al>Na\]
done
clear
View Answer play_arrow
question_answer 151) Which of the following statements is correct?
A)
\[{{X}^{-}}\] ion is larger in size than X atom
done
clear
B)
\[{{X}^{+}}\] ion is larger in size than X atom
done
clear
C)
\[{{X}^{+}}\] ion is larger in size than \[{{X}^{-}}\] ion
done
clear
D)
\[{{X}^{+}}\] and \[{{X}^{-}}\] ions are equal in size
done
clear
View Answer play_arrow
question_answer 152) The correct order of electron affinity of B, C, N, O is
A)
\[O>C>N>B\]
done
clear
B)
\[B>N>C>O\]
done
clear
C)
\[\text{O}>C>B>N\]
done
clear
D)
\[\text{O}>B>C>N\]
done
clear
View Answer play_arrow
question_answer 153) The ionic radius of which of the following would be maximum?
A)
\[{{C}^{4-}}\]
done
clear
B)
\[{{N}^{3-}}\]
done
clear
C)
\[{{O}^{2-}}\]
done
clear
D)
\[M{{g}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 154) The atomic radius of elements of which of the following series would be nearly the same?
A)
\[Na,K,Rb,Cs\]
done
clear
B)
\[Li,Be,B,C\]
done
clear
C)
\[Fe,Co,Ni,Cu~\]
done
clear
D)
\[F,Cl,Br,I\]
done
clear
View Answer play_arrow
question_answer 155) Gradual addition of electronic shells in the noble gases causes a decrease in their
A)
ionisation energy
done
clear
B)
atomic radius
done
clear
C)
boiling point
done
clear
D)
density
done
clear
View Answer play_arrow
question_answer 156) Monazite is a source of
A)
\[Ne\]
done
clear
B)
\[Ra\]
done
clear
C)
\[Kr\]
done
clear
D)
\[Th\]
done
clear
View Answer play_arrow
question_answer 157) Which forms coloured ion?
A)
Metalloids
done
clear
B)
Non-metals
done
clear
C)
p-block elements
done
clear
D)
Transition elements
done
clear
View Answer play_arrow
question_answer 158) Prussian blue is
A)
\[F{{e}_{4}}{{[Fe{{(CN)}_{6}}]}_{3}}\]
done
clear
B)
\[F{{e}_{2}}[Fe{{(CN)}_{6}}]\]
done
clear
C)
\[F{{e}_{3}}[Fe{{(CN)}_{6}}]\]
done
clear
D)
\[Fe{{[Fe{{(CN)}_{6}}]}_{2}}\]
done
clear
View Answer play_arrow
question_answer 159) The highest oxidation state is shown by that transition element which has outermost configuration as
A)
\[{{d}^{3}}{{s}^{2}}\]
done
clear
B)
\[{{d}^{5}}{{s}^{1}}\]
done
clear
C)
\[{{d}^{5}}{{s}^{2}}\]
done
clear
D)
\[{{d}^{8}}{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 160) Which of the following oxides is white but becomes yellow on heating?
A)
\[AgO\]
done
clear
B)
\[A{{g}_{2}}O\]
done
clear
C)
\[FeO\]
done
clear
D)
\[ZnO\]
done
clear
View Answer play_arrow
question_answer 161) Which of the following ions gives coloured solution?
A)
\[C{{u}^{+}}\]
done
clear
B)
\[Z{{n}^{2+}}\]
done
clear
C)
\[A{{g}^{+}}\]
done
clear
D)
\[F{{e}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 162) On heating \[Mn{{(OH)}_{2}}\] with \[Pb{{O}_{2}}\]and cone. \[HN{{O}_{3}}\] pink colour is obtained due to the formation of
A)
\[KMn{{O}_{4}}\]
done
clear
B)
\[HMn{{O}_{4}}\]
done
clear
C)
\[Pb{{(Mn{{O}_{4}})}_{2}}\]
done
clear
D)
\[PbMn{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 163) On the basis of Le-Chatelier's principle, predict which of the following conditions would be unfavorable for the formation of \[S{{O}_{3}}\]? Given that: \[2S{{O}_{2}}+{{O}_{2}}\rightleftharpoons 2S{{O}_{3}}\,\,\Delta H=-42kcal\]
A)
Low temperature
done
clear
B)
High pressure
done
clear
C)
High temperature
done
clear
D)
High concentration of \[S{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 164) An element \[(X)\] forms compounds of the formulae \[XC{{l}_{3}},\] \[{{X}_{2}}{{O}_{5}}\] and \[C{{a}_{3}}{{X}_{2}}\] but does not form \[XC{{l}_{5}}\]. Which of the following is the element X?
A)
\[B\]
done
clear
B)
\[Al\]
done
clear
C)
\[N\]
done
clear
D)
\[P\]
done
clear
View Answer play_arrow
question_answer 165) Which of the following oxides of nitrogen reacts with ferrous sulphate to form a dark brown compound?
A)
\[{{N}_{2}}O\]
done
clear
B)
\[NO\]
done
clear
C)
\[N{{O}_{2}}\]
done
clear
D)
\[{{N}_{2}}{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 166) Which of the following halogens does not form oxyacid?
A)
Fluorine
done
clear
B)
Chlorine
done
clear
C)
Bromine
done
clear
D)
Iodine
done
clear
View Answer play_arrow
question_answer 167) The strongest of the following acids, is
A)
\[HBr\]
done
clear
B)
\[HCl\]
done
clear
C)
\[HF\]
done
clear
D)
\[HI\]
done
clear
View Answer play_arrow
question_answer 168) Nessler's reagent is
A)
\[KHg{{I}_{4}}\]
done
clear
B)
\[{{K}_{2}}Hg{{I}_{4}}+N{{H}_{4}}OH\]
done
clear
C)
\[{{K}_{2}}Hg{{I}_{4}}+KOH\]
done
clear
D)
\[KHg{{I}_{4}}+N{{H}_{4}}OH\]
done
clear
View Answer play_arrow
question_answer 169) Helium is used with oxygen in the apparatus of divers because it
A)
is lighter than nitrogen
done
clear
B)
is not soluble in blood at high pressure
done
clear
C)
is easily available
done
clear
D)
is less reactive than nitrogen
done
clear
View Answer play_arrow
question_answer 170) Which of the following is a favourable facer ' for cation formation?
A)
Low ionisation potential
done
clear
B)
High electron affinity
done
clear
C)
High electronegativity
done
clear
D)
Small atomic size
done
clear
View Answer play_arrow
question_answer 171) Very dilute nitric add reacts with zinc to far- zinc nitrate and
A)
amonium nitrate
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[NO\]
done
clear
D)
\[~{{N}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 172) Unlike other halogens, fluorine does not show higher oxidation states, because
A)
it is highly electronegative
done
clear
B)
it has no d-orbitals
done
clear
C)
its atomic radius is very small
done
clear
D)
The \[{{F}^{-}}\] ion is stable and isoelectronic with neon
done
clear
View Answer play_arrow
question_answer 173) Which of the following hydrides has the lowest boiling point?
A)
\[{{H}_{2}}O\]
done
clear
B)
\[{{H}_{2}}S\]
done
clear
C)
\[{{H}_{2}}Se\]
done
clear
D)
\[{{H}_{2}}Te\]
done
clear
View Answer play_arrow
question_answer 174) The number of isomers possible for square planar complex \[{{K}_{2}}[PdClB{{r}_{2}}(SCN)]\] is
A)
\[2\]
done
clear
B)
\[3\]
done
clear
C)
\[4\]
done
clear
D)
\[6\]
done
clear
View Answer play_arrow
question_answer 175) IUPAC name of \[[Co{{(N{{H}_{3}})}_{3}}({{H}_{2}}O)Cl]C{{l}_{2}}\]is
A)
Diaquachlorodiaminecobalt (III) chloride
done
clear
B)
Triaminediaquachlorocobalt (III) chloride
done
clear
C)
Chlorodiaminediaquacobalt (II) chloride
done
clear
D)
Diaminediaquachlorocobalt (II) chloride
done
clear
View Answer play_arrow
question_answer 176) Which of the following exhibits highest molar conductivity?
A)
\[[Co{{(N{{H}_{3}})}_{6}}]C{{l}_{3}}\]
done
clear
B)
\[[Co{{(N{{H}_{3}})}_{5}}Cl]C{{l}_{2}}\]
done
clear
C)
\[[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]Cl\]
done
clear
D)
\[[Co{{(N{{H}_{3}})}_{3}}C{{l}_{3}}]\]
done
clear
View Answer play_arrow
question_answer 177) In tris (ethylene diamine) cobalt (III) chloride the coordination number of cobalt is
A)
\[3\]
done
clear
B)
\[4\]
done
clear
C)
\[6\]
done
clear
D)
\[7\]
done
clear
View Answer play_arrow
question_answer 178) The ion that can be precipitated by \[HCl\] as well as \[{{H}_{2}}S\] is
A)
\[P{{b}^{2+}}\]
done
clear
B)
\[F{{e}^{3+}}\]
done
clear
C)
\[Z{{n}^{2+}}\]
done
clear
D)
\[C{{u}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 179) A salt on treatment with dil. \[HCl\] give: pungent smelling gas and a yellow precipitate The salt gives green flame when tested. The salt solution gives a yellow precipitate with potassium chromate. The salt is
A)
\[NiS{{O}_{4}}\]
done
clear
B)
\[Ba{{S}_{2}}{{O}_{3}}\]
done
clear
C)
\[Pb{{S}_{2}}{{O}_{3}}\]
done
clear
D)
\[CuS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 180) Which of the following is not used as an explosive?
A)
Trinitrotoluene
done
clear
B)
Trinitrobenzene
done
clear
C)
Picric acid
done
clear
D)
Nitrobenzene
done
clear
View Answer play_arrow
question_answer 181) Which of the following will have geometrical isomers?
A)
2-methylpropene
done
clear
B)
2-butene
done
clear
C)
1-butene
done
clear
D)
Propene
done
clear
View Answer play_arrow
question_answer 182) Which statement is true for cyclohexane?
A)
It has two possible isomers
done
clear
B)
It has three conformations
done
clear
C)
Boat conformation is most stable
done
clear
D)
Chair and boat conformations differ in energy by \[44\text{ }kJ/mol\]
done
clear
View Answer play_arrow
question_answer 183) A hydrocarbon has \[C\text{ }85.72%\]and remaining H. The hydrocarbon is
A)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
D)
\[C{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 184) Which of the following has highest octane number?
A)
n-hexane
done
clear
B)
n-heptane
done
clear
C)
iso-octane
done
clear
D)
n-heptane and iso-octane mixed in ratio \[50:50\]
done
clear
View Answer play_arrow
question_answer 185) Which of the following is obtained at lowest temperature by fractional distillation of petroleum?
A)
Ligroin
done
clear
B)
Gasoline
done
clear
C)
Kerosene
done
clear
D)
Diesel oil
done
clear
View Answer play_arrow
question_answer 186) The order of stability of carbonium ions is
A)
\[methyl>ethyl>iso-propyl>tert-butyl\]
done
clear
B)
\[tert-butyl>iso-propyl>ethyl>methyl\]
done
clear
C)
\[iso-propyl>tert-butyl>ethyl>methyl\]
done
clear
D)
\[tert-butyl>ethyl>iso-propyl>methyl\]
done
clear
View Answer play_arrow
question_answer 187) The compound which gives only acetaldehyde on ozonolysis is
A)
butene-1
done
clear
B)
butene-2
done
clear
C)
ethylene
done
clear
D)
propylene
done
clear
View Answer play_arrow
question_answer 188) Which of the following is used to distinguish ethylene and acetylene?
A)
Alkaline \[KMn{{O}_{4}}\]
done
clear
B)
Bromine water
done
clear
C)
Ammoniacal cuprous chloride
done
clear
D)
Conc.\[{{H}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 189) Carbons in the compound l-butene-3-yne are
A)
\[sp\] hybridized
done
clear
B)
\[s{{p}^{2}}\]hybridized
done
clear
C)
\[sp\] and \[s{{p}^{2}}\] hybridized
done
clear
D)
\[sp,\] \[s{{p}^{2}}\]and \[s{{p}^{3}}\]hybridized
done
clear
View Answer play_arrow
question_answer 190) In nitration of benzene with cone. \[HN{{O}_{3}}\] and cone. \[{{H}_{2}}S{{O}_{4}},\] the species which takes active part is
A)
nitrite ion
done
clear
B)
nitrate ion
done
clear
C)
nitroniumion
done
clear
D)
nitrogen peroxide
done
clear
View Answer play_arrow
question_answer 191) Ethyl bromide on treatment with alcoholic \[KOH\] gives
A)
ethylene
done
clear
B)
ethanol
done
clear
C)
acetic acid
done
clear
D)
ethane
done
clear
View Answer play_arrow
question_answer 192) \[AgN{{O}_{3}}\] does not give precipitate with chloroform because
A)
\[CHC{{1}_{3}}\]is insoluble in water
done
clear
B)
\[CHC{{1}_{3}}\]does not ionise in water
done
clear
C)
\[CHC{{1}_{3}}\] is an organic compound
done
clear
D)
\[AgN{{O}_{3}}\]is insoluble in \[CHC{{1}_{3}}\]
done
clear
View Answer play_arrow
question_answer 193) Benzene reacts with chlorine to form benzene hex chloride in presence of
A)
nickel
done
clear
B)
\[AlC{{l}_{3}}\]
done
clear
C)
bright sunlight
done
clear
D)
zinc
done
clear
View Answer play_arrow
question_answer 194) Which of the following compounds gives a ketone with Grignard reagent?
A)
Formaldehyde
done
clear
B)
Ethyl alcohol
done
clear
C)
Methyl cyanide
done
clear
D)
Methyl iodide
done
clear
View Answer play_arrow
question_answer 195) Which one of the following compounds gives a positive iodofonn test?
A)
Pentanal
done
clear
B)
1-phenyl ethanol
done
clear
C)
2-phenyl ethanol
done
clear
D)
3-pentanol
done
clear
View Answer play_arrow
question_answer 196) Reaction of acetaldehyde with \[HCN\] followed by hydrolysis gives a compound which shows
A)
optical isomerism
done
clear
B)
geometrical isomerism
done
clear
C)
metamerism
done
clear
D)
tautomerism
done
clear
View Answer play_arrow
question_answer 197) A compound A has a molecular formula \[{{C}_{2}}C{{l}_{3}}OH\]. It reduces Fehling solution and on oxidation gives a monocarboxylic acid .A is obtained by action of chlorine on ethyl alcohol. A is
A)
chloral
done
clear
B)
\[C{{H}_{3}}Cl\]
done
clear
C)
\[C{{H}_{3}}Cl\]
done
clear
D)
chloroacetic acid
done
clear
View Answer play_arrow
question_answer 198) Which of the following is not a synthetic polymer?
A)
Polyethylene
done
clear
B)
PVC
done
clear
C)
Nylon
done
clear
D)
Cellophane
done
clear
View Answer play_arrow
question_answer 199) Iodine value is related to
A)
fats and oils
done
clear
B)
alcohols
done
clear
C)
esters
done
clear
D)
hydrocarbons
done
clear
View Answer play_arrow
question_answer 200) Deficiency of which vitamin causes rickets?
A)
Vitamin C
done
clear
B)
Vitamin D
done
clear
C)
Vitamin A
done
clear
D)
Vitamin K
done
clear
View Answer play_arrow
question_answer 201) Epithelial tissue serves as
A)
protective sheath
done
clear
B)
reproductive structures
done
clear
C)
corpuscle
done
clear
D)
nerve cells
done
clear
View Answer play_arrow
question_answer 202) Muscular tissue is differentiated into
A)
unstriped, striped
done
clear
B)
striped, cardiac
done
clear
C)
unstriped, cardiac muscle
done
clear
D)
unstriped, striated and cardiac
done
clear
View Answer play_arrow
question_answer 203) The units of the nervous system are
A)
neurons
done
clear
B)
dendrites
done
clear
C)
axon
done
clear
D)
cyton
done
clear
View Answer play_arrow
question_answer 204) The hardest substance of vertebrate body is
A)
keratin
done
clear
B)
enamel
done
clear
C)
dentine
done
clear
D)
chondrin
done
clear
View Answer play_arrow
question_answer 205) Role of bone marrow in mammals is
A)
to assist kidneys
done
clear
B)
to act as haemopoietic tissue
done
clear
C)
to assist liver
done
clear
D)
to control blood pressure
done
clear
View Answer play_arrow
question_answer 206) Which of these contains vocal cords?
A)
Pharynx
done
clear
B)
Larynx
done
clear
C)
Glottis
done
clear
D)
Bronchial tube
done
clear
View Answer play_arrow
question_answer 207) Which of the following glands is present in frog's skin and not in rabbit's skin?
A)
Sebaceous gland
done
clear
B)
Sweat gland
done
clear
C)
Mucous gland
done
clear
D)
Moll's gland
done
clear
View Answer play_arrow
question_answer 208) How many kinds of cells are found in islets of Langerhans?
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 209) Which type of cells secretes \[\text{HCl}\] in stomach'
A)
Chief cells
done
clear
B)
Oxyntic cells
done
clear
C)
Peneth cells
done
clear
D)
Goblet cells
done
clear
View Answer play_arrow
question_answer 210) The pungent odour of faeces is due to ne presence of
A)
indole and skatole
done
clear
B)
skatole only
done
clear
C)
phenol and hydrogen sulphide
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 211) Which one of the following enzymes initials protein digestion?
A)
Amino peptidase
done
clear
B)
Carboxy peptidase
done
clear
C)
Trypsin
done
clear
D)
Pepsin
done
clear
View Answer play_arrow
question_answer 212) Haemoglobin is having maximum affinity with
A)
\[C{{C}_{2}}\]
done
clear
B)
CO
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 213) In our body, the blood bank is
A)
red bone marrow
done
clear
B)
spleen
done
clear
C)
liver
done
clear
D)
heart
done
clear
View Answer play_arrow
question_answer 214) Down's syndrome is
A)
autosomal abnormality
done
clear
B)
sex chromosomal abnormality
done
clear
C)
sex-linked disease
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 215) The wall of heart is made up of
A)
epicardium
done
clear
B)
myocardium
done
clear
C)
endocardium
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 216) Carbonic anhydrase is present in high concentrations in
A)
plasma
done
clear
B)
erythrocytes
done
clear
C)
leucocytes
done
clear
D)
nephrons
done
clear
View Answer play_arrow
question_answer 217) How many molecules of oxygen can bound to one molecule of haemoglobin?
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 218) Why do we pass more urine in wet and cold season?
A)
Impairment of water absorption by nephrons
done
clear
B)
Kidney becomes more active
done
clear
C)
Sweating is much reduced
done
clear
D)
ADH secretion is increased
done
clear
View Answer play_arrow
question_answer 219) What for the ascending limb of loop of Henie is permeable?
A)
Glucose
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
Na
done
clear
D)
Water
done
clear
View Answer play_arrow
question_answer 220) Which is the correct explanatory word for the composition of protoplasm?
A)
Emulsion
done
clear
B)
Suspension
done
clear
C)
Complex colloidal solution
done
clear
D)
Molecular solution
done
clear
View Answer play_arrow
question_answer 221) Which part of mammalian brain controls the muscular co-ordination?
A)
Cerebrum
done
clear
B)
Cerebellum
done
clear
C)
Corpus callosum
done
clear
D)
Medulla
done
clear
View Answer play_arrow
question_answer 222) Name of nervous band connecting cerebral hemispheres in rabbit is
A)
corpus callosum
done
clear
B)
corpus albicans
done
clear
C)
corpus striatum
done
clear
D)
corpus spongiosum
done
clear
View Answer play_arrow
question_answer 223) Lateral ventricles are found in
A)
heart
done
clear
B)
brain
done
clear
C)
thyroid
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 224) In which bee barbless sting is found?
A)
Drones
done
clear
B)
Workers
done
clear
C)
Queen bee
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 225) When are the identical twins born?
A)
One sperm fertilizes two ova
done
clear
B)
One ovum is fertilized by two sperms
done
clear
C)
Two ova are fertilized by two sperms
done
clear
D)
One fertilized ovum divides into two blastomeres and both of them separate
done
clear
View Answer play_arrow
question_answer 226) One mole glucose, on complete oxidation, yields energy
A)
686 cal
done
clear
B)
68,600 cal
done
clear
C)
6,86,000 cal
done
clear
D)
68,60,000 cal
done
clear
View Answer play_arrow
question_answer 227) The points of sperm entry during fertilization forms
A)
centre of rotation of embryo
done
clear
B)
axis of cleavage
done
clear
C)
grey crescent
done
clear
D)
dorsal lip of blastopore
done
clear
View Answer play_arrow
question_answer 228) Seminiferous tubules are found in
A)
testis
done
clear
B)
ovary
done
clear
C)
kidney
done
clear
D)
lung
done
clear
View Answer play_arrow
question_answer 229) Corpus luteum is formed in
A)
ovary
done
clear
B)
testis
done
clear
C)
mammary gland
done
clear
D)
uterus
done
clear
View Answer play_arrow
question_answer 230) Fimbriated funnel is
A)
proximal part of oviduct
done
clear
B)
uterus part
done
clear
C)
urinary bladder part
done
clear
D)
ureter part
done
clear
View Answer play_arrow
question_answer 231) Oogenesis comprises
A)
multiplication phase
done
clear
B)
growth phase
done
clear
C)
maturation phase
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 232) Acrosome is found in the sperm at
A)
top part
done
clear
B)
middle piece
done
clear
C)
behind the nucleus
done
clear
D)
tail part
done
clear
View Answer play_arrow
question_answer 233) In mammals, the number of cervical vertebra is
A)
5
done
clear
B)
7
done
clear
C)
10
done
clear
D)
12
done
clear
View Answer play_arrow
question_answer 234) In mammals, the lower jaw is made up of
A)
dentaries
done
clear
B)
maxilla
done
clear
C)
premaxilla
done
clear
D)
palatine
done
clear
View Answer play_arrow
question_answer 235) In mammals, each half of pectoral girdle consists of
A)
supra scapula
done
clear
B)
scapula
done
clear
C)
coracoid
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 236) In mammals, each half of pelvic girdle consists of
A)
ilium
done
clear
B)
ischium
done
clear
C)
pubis
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 237) Ear drum is known as
A)
tympanic membrane
done
clear
B)
tensor tympani
done
clear
C)
scala tympani
done
clear
D)
scala vestibule
done
clear
View Answer play_arrow
question_answer 238) Function of lacrimal glands is
A)
to secrete mucous
done
clear
B)
to secrete tears
done
clear
C)
to secrete oil
done
clear
D)
to secrete fat
done
clear
View Answer play_arrow
question_answer 239) Life saving hormone is secreted by which gland?
A)
Adrenal gland
done
clear
B)
Hypothalamus
done
clear
C)
Pituitary gland
done
clear
D)
Thyroid gland
done
clear
View Answer play_arrow
question_answer 240) The disease caused by deficiency of iodine is
A)
cretinism
done
clear
B)
myxedema
done
clear
C)
goitre
done
clear
D)
tetany
done
clear
View Answer play_arrow
question_answer 241) The disease caused by deficiency of parathormone is
A)
cretinism
done
clear
B)
tetany
done
clear
C)
hypercalemia
done
clear
D)
myxoedema
done
clear
View Answer play_arrow
question_answer 242) Cretinism is due to less secretion of
A)
thyroid gland
done
clear
B)
pituitary gland
done
clear
C)
parathyroid gland
done
clear
D)
adrenal gland
done
clear
View Answer play_arrow
question_answer 243) Which endocrine gland becomes inactive in old age?
A)
adrenal
done
clear
B)
pineal
done
clear
C)
thymus
done
clear
D)
pituitary
done
clear
View Answer play_arrow
question_answer 244) In males, the essential hormone for secondary sexual characteristics is
A)
testosterone
done
clear
B)
progesterone
done
clear
C)
estrogen
done
clear
D)
relaxin
done
clear
View Answer play_arrow
question_answer 245) In females, the essential hormone for secondary sexual characteristics is
A)
testosterone
done
clear
B)
progesterone
done
clear
C)
estrogen
done
clear
D)
relaxin
done
clear
View Answer play_arrow
question_answer 246) Pellagra disease is caused due to deficiency of vitamin
A)
B
done
clear
B)
C
done
clear
C)
D
done
clear
D)
E
done
clear
View Answer play_arrow
question_answer 247) Submucosa is thickest in
A)
stomach
done
clear
B)
oesophagus
done
clear
C)
intestine
done
clear
D)
rectum
done
clear
View Answer play_arrow
question_answer 248) Mineral deposition in bones and teeth is due to vitamin
A)
D
done
clear
B)
A
done
clear
C)
C
done
clear
D)
E
done
clear
View Answer play_arrow
question_answer 249) Which is the urinary bladder of the child placed in the womb?
A)
Urinary bladder
done
clear
B)
Amnion
done
clear
C)
Allantois
done
clear
D)
Liver
done
clear
View Answer play_arrow
question_answer 250) What is the cause of haemophilia?
A)
Chromosomal aberration
done
clear
B)
Somatic mutation
done
clear
C)
X-linked mutation
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 251) The term blastocyst is applied to the blastula of which one of the following?
A)
Kangaroo
done
clear
B)
Platypus
done
clear
C)
Monkey
done
clear
D)
Both [a] and [c]
done
clear
View Answer play_arrow
question_answer 252) The outer layer of the blastocyst which forms the ectoderm, is called
A)
cnidoblast
done
clear
B)
germinal vesicle
done
clear
C)
trophoblast
done
clear
D)
amnion
done
clear
View Answer play_arrow
question_answer 253) The foetal membrane, which is the source of first blood corpuscle to enter the circulation the embryo, is called
A)
amnion
done
clear
B)
chorion
done
clear
C)
trophoblast
done
clear
D)
yolk sac
done
clear
View Answer play_arrow
question_answer 254) Growth in most warm blooded vertebrates is
A)
determinate
done
clear
B)
indeterminate
done
clear
C)
uncontrolled
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 255) The ability of animals to regenerate the lost parts of the body was reported by
A)
Carlson
done
clear
B)
Trembley
done
clear
C)
Patten
done
clear
D)
Storer
done
clear
View Answer play_arrow
question_answer 256) Improvement of genetic characters and present day generation on the basis of best nutrition and training is called
A)
Eugenics
done
clear
B)
Euphenics
done
clear
C)
Euthenics
done
clear
D)
Gerontology
done
clear
View Answer play_arrow
question_answer 257) The 'Cell theory" states that
A)
all cells have nuclei
done
clear
B)
cells are the fundamental structural mat of plants and animals
done
clear
C)
all cells are living
done
clear
D)
cells reproduce by mitosis and meiosis
done
clear
View Answer play_arrow
question_answer 258) Structure present over the chromosomes is
A)
nucleolus
done
clear
B)
centromere
done
clear
C)
centrosome
done
clear
D)
Golgi complex
done
clear
View Answer play_arrow
question_answer 259) Normally the number of chromosomes in the nuclei of gametes that fuse at fertilization are
A)
innumerable
done
clear
B)
dissimilar
done
clear
C)
similar
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 260) Balbiani discovered special type of chromosome from the salivary gland of Chironomus larva, which are recognized by the presence of
A)
bands
done
clear
B)
loops
done
clear
C)
Both [a] and [b]
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 261) The technique which makes use of amniotic fluid for the detection of foetal disorders, is called as
A)
endoscopy
done
clear
B)
laproscopy
done
clear
C)
natal endoscopy
done
clear
D)
amniocentesis
done
clear
View Answer play_arrow
question_answer 262) Which of the following is not related to chromosomal aberration?
A)
Euploidy
done
clear
B)
AIDS
done
clear
C)
Aneuploidy
done
clear
D)
Klinefelter's syndrome
done
clear
View Answer play_arrow
question_answer 263) Immediately after ovulation, the mammalian egg is covered by an outermost membrane, called as
A)
vitelline membrane
done
clear
B)
chorion
done
clear
C)
zona pellucid
done
clear
D)
corona radiate
done
clear
View Answer play_arrow
question_answer 264) The number of autosomes in man is
A)
22 pairs
done
clear
B)
11 pairs
done
clear
C)
43 pairs
done
clear
D)
23 pairs
done
clear
View Answer play_arrow
question_answer 265) Supermale and super female type determination of sex in Drosophila is base on
A)
genie balance
done
clear
B)
oxygen balance
done
clear
C)
biodiversity
done
clear
D)
uniformity
done
clear
View Answer play_arrow
question_answer 266) A girl of normal vision whose father was colourblind marries a man of normal vision whose father was also colourblind. Their sons would be (of total number of sons).
A)
all colourblind
done
clear
B)
50% colourblind
done
clear
C)
all normal
done
clear
D)
25% colourblind
done
clear
View Answer play_arrow
question_answer 267) Marriage between one of the following pairs may cause child death
A)
\[R{{h}^{+}}\] father and \[R{{h}^{-}}\]mother
done
clear
B)
\[K{{h}^{+}}\] father and \[R{{h}^{+}}\]mother
done
clear
C)
\[R{{h}^{-}}\] father and \[R{{h}^{-}}\] mother
done
clear
D)
\[R{{h}^{-}}\] father and \[R{{h}^{+}}\]mother
done
clear
View Answer play_arrow
question_answer 268) Donors and recipients in a blood transfusion process can be
A)
only father and son
done
clear
B)
only brother and sister
done
clear
C)
only maternal uncle and niece
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 269) Which one of the following is not a mutagen?
A)
Acetic acid
done
clear
B)
Hydroxylamine
done
clear
C)
Nitrous acid
done
clear
D)
Gamma rays
done
clear
View Answer play_arrow
question_answer 270) The enzyme which can cut molecules of DNA into segments is known as
A)
DNA polymerase
done
clear
B)
DNA ligase
done
clear
C)
Restriction enzyme
done
clear
D)
DNA gyrase
done
clear
View Answer play_arrow
question_answer 271) Point mutation is a change, which involves
A)
loss of a gene
done
clear
B)
addition of a gene
done
clear
C)
deletion of a segment of a gene
done
clear
D)
change in a base of a gene
done
clear
View Answer play_arrow
question_answer 272) The percentage ratio of natality over mortality is called
A)
vital index
done
clear
B)
population density
done
clear
C)
total count of individuals
done
clear
D)
fertility rate
done
clear
View Answer play_arrow
question_answer 273) Which is non-poisonous?
A)
Spider
done
clear
B)
Scorpion
done
clear
C)
Centipede
done
clear
D)
Crab
done
clear
View Answer play_arrow
question_answer 274) Electric organs are found in
A)
sharks
done
clear
B)
porpoises
done
clear
C)
goldfish
done
clear
D)
rays
done
clear
View Answer play_arrow
question_answer 275) Which monkey has prehensile tail?
A)
Spider monkey
done
clear
B)
Semnopithecus
done
clear
C)
Rhesus monkey
done
clear
D)
Boinnet monkey
done
clear
View Answer play_arrow
question_answer 276) Which of the following is in tracheate group?
A)
Crab-centipede-cockroach
done
clear
B)
King Crab-scorpion-housefly
done
clear
C)
Spider-Peripatus-mosquito
done
clear
D)
Bedbug-sandfly-silkworm
done
clear
View Answer play_arrow
question_answer 277) Which is filter feeder?
A)
Amoeba
done
clear
B)
Leech
done
clear
C)
Spider
done
clear
D)
Paramedum
done
clear
View Answer play_arrow
question_answer 278) Whose skin colour does not change?
A)
Chamaeleon
done
clear
B)
Horse
done
clear
C)
Garden lizard
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 279) Phylogeny tells about
A)
life history of animals
done
clear
B)
group of phyla
done
clear
C)
evolutionary history of a species from its ancestors
done
clear
D)
castes of flies
done
clear
View Answer play_arrow
question_answer 280) Which one of the following scientists is not related with organic evolution?
A)
Erasmus Darwin
done
clear
B)
Charles Darwin
done
clear
C)
Darlington
done
clear
D)
TR Malthus
done
clear
View Answer play_arrow
question_answer 281) Modemtheory of organic evolution is based on
A)
population
done
clear
B)
mutation
done
clear
C)
isolation
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 282) A vestigial organ of man is
A)
vermiform appendix
done
clear
B)
sebaceous gland
done
clear
C)
pelvic girdle
done
clear
D)
mammary gland
done
clear
View Answer play_arrow
question_answer 283) The wings of an insect and a bat exhibit
A)
homology
done
clear
B)
analogy
done
clear
C)
atavism
done
clear
D)
connecting link
done
clear
View Answer play_arrow
question_answer 284) The material for organic evolution is
A)
effect of hormones
done
clear
B)
nutritive value
done
clear
C)
mutation
done
clear
D)
asexual reproduction
done
clear
View Answer play_arrow
question_answer 285) The nearest prehistoric ancestor of present man may be
A)
Java ape man
done
clear
B)
Cro-magnon man
done
clear
C)
Neanderthal man
done
clear
D)
Peking man
done
clear
View Answer play_arrow
question_answer 286) Silkworm is a
A)
fly
done
clear
B)
worm
done
clear
C)
moth
done
clear
D)
beetle
done
clear
View Answer play_arrow
question_answer 287) Who is referred to as father of pearl industry ?
A)
Ivanowski
done
clear
B)
Louis Pasteur
done
clear
C)
Kokichi Mikimoto
done
clear
D)
Harvey
done
clear
View Answer play_arrow
question_answer 288) Plasmodium in man is inoculated by
A)
Anopheles male
done
clear
B)
Anopheles female
done
clear
C)
Culex female
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 289) 'Malaria', a common disease worldwide, b caused by a
A)
bacterium
done
clear
B)
virion
done
clear
C)
Protozoa
done
clear
D)
helminthes
done
clear
View Answer play_arrow
question_answer 290) HIV causes reduction in
A)
T-helper cells only
done
clear
B)
All T-cells
done
clear
C)
B-cells only
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 291) Biological name of insect (vector) carrying the plague is
A)
Xenopsylla cheopis
done
clear
B)
Anopheles mosquito
done
clear
C)
Bacillus pestis
done
clear
D)
Pediculus humanus
done
clear
View Answer play_arrow
question_answer 292) A molecule that elicites an immune response is called
A)
antibody
done
clear
B)
antigen
done
clear
C)
mutagen
done
clear
D)
carcinogen
done
clear
View Answer play_arrow
question_answer 293) Malarial parasite completes its life-cycle in
A)
one host
done
clear
B)
two hosts
done
clear
C)
three hosts
done
clear
D)
reservoir host
done
clear
View Answer play_arrow
question_answer 294) Metabolic waste responsible for malaria fever is called
A)
haemozoin
done
clear
B)
haematin
done
clear
C)
melanin
done
clear
D)
heparin
done
clear
View Answer play_arrow
question_answer 295) A pearl oyster secretes pearls to
A)
regenerate injured parts
done
clear
B)
protect itself against invading parasite
done
clear
C)
harden its mantle cavity
done
clear
D)
isolate damaged tissues of the body
done
clear
View Answer play_arrow
question_answer 296) Addiction to tobacco is caused by the presence of
A)
caffiene
done
clear
B)
nicotine
done
clear
C)
cocaine
done
clear
D)
histamine
done
clear
View Answer play_arrow
question_answer 297) Which of the following organ is most affected by alcohol?
A)
Kidney
done
clear
B)
Heart
done
clear
C)
Liver
done
clear
D)
Lungs
done
clear
View Answer play_arrow
question_answer 298) Kanha National Park is famous for
A)
birds
done
clear
B)
rhinoceros
done
clear
C)
tigers
done
clear
D)
crocodiles
done
clear
View Answer play_arrow
question_answer 299) Malathion, parathion and fenitrothion belong to the group of
A)
carbamates
done
clear
B)
organophosphates
done
clear
C)
pyrethroids
done
clear
D)
marines
done
clear
View Answer play_arrow
question_answer 300) Which of the following when dissolved in water make (s) Bordeaux mixture?
A)
Copper sulphate
done
clear
B)
Calcium oxide
done
clear
C)
Both [a] and [b]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 301) The significance of meiosis lies in
A)
maintaining constancy in the number of chromosomes in an organism
done
clear
B)
production of genetic variability in the population of species
done
clear
C)
Reduction of the diploid number of chromosomes to haploid
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 302) Which among the following can be seen only under the electron microscope?
A)
Chloroplast
done
clear
B)
Ribosome
done
clear
C)
Leucoplast
done
clear
D)
Nucleus
done
clear
View Answer play_arrow
question_answer 303) The name 'protoplasm' was given by
A)
Hooke
done
clear
B)
A K Sharma
done
clear
C)
Schwann
done
clear
D)
Purkinje
done
clear
View Answer play_arrow
question_answer 304) A mature plant cell has
A)
cell wall and protoplasm
done
clear
B)
protoplasm and vacuole
done
clear
C)
vacuole and cell wall
done
clear
D)
Both [b] and [c]
done
clear
View Answer play_arrow
question_answer 305) The larger sub-unit in 80S ribosome is
A)
50S
done
clear
B)
60S
done
clear
C)
40S
done
clear
D)
0S
done
clear
View Answer play_arrow
question_answer 306) Golgi bodies are absent in
A)
plants
done
clear
B)
bacteria
done
clear
C)
animals
done
clear
D)
eukaryotic cells
done
clear
View Answer play_arrow
question_answer 307) Endoplasmic reticulum is more developed in
A)
green cells
done
clear
B)
young cells
done
clear
C)
mature cells
done
clear
D)
bacteriophage
done
clear
View Answer play_arrow
question_answer 308) Mitochondria are related to
A)
prokaryotes
done
clear
B)
plasmids
done
clear
C)
plastids
done
clear
D)
viruses
done
clear
View Answer play_arrow
question_answer 309) The main function of lysosomes is
A)
digestion
done
clear
B)
replication
done
clear
C)
translation
done
clear
D)
translocation
done
clear
View Answer play_arrow
question_answer 310) The physical basis of life is
A)
nucleus
done
clear
B)
sex chromosomes
done
clear
C)
protoplasm
done
clear
D)
DNA
done
clear
View Answer play_arrow
question_answer 311) Which of the following has a single membrane?
A)
Ribosome
done
clear
B)
Peroxisome
done
clear
C)
Nucleolus
done
clear
D)
Centrosome
done
clear
View Answer play_arrow
question_answer 312) DNA molecules of each chromosome become double in
A)
\[{{\text{G}}_{\text{1}}}\text{-}\]phase
done
clear
B)
\[{{\text{G}}_{2}}\text{-}\]phase
done
clear
C)
S-phase
done
clear
D)
M-phase
done
clear
View Answer play_arrow
question_answer 313) L-shaped chromosomes are called
A)
sex-chromosome
done
clear
B)
acrocentric
done
clear
C)
telocentric
done
clear
D)
sub-metacentric
done
clear
View Answer play_arrow
question_answer 314) Pairing of homologous chromosomes takes place in
A)
pachytene
done
clear
B)
zygotene
done
clear
C)
diplotene
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 315) How many meiotic divisions will be necessary to produce two hundred pollen grains?
A)
100
done
clear
B)
199
done
clear
C)
50
done
clear
D)
200
done
clear
View Answer play_arrow
question_answer 316) Which of the following is a purine ?
A)
Prokaryotes
done
clear
B)
Guanine
done
clear
C)
Thymine
done
clear
D)
Uracil
done
clear
View Answer play_arrow
question_answer 317) Which type of ribosomes are found in Nostoc cells?
A)
50S
done
clear
B)
60S
done
clear
C)
70S
done
clear
D)
Eukaryotic
done
clear
View Answer play_arrow
question_answer 318) A change in the chromosome number is called
A)
chromosomal mutation
done
clear
B)
gene mutation
done
clear
C)
somatic mutation
done
clear
D)
polyploidy
done
clear
View Answer play_arrow
question_answer 319) Who coined the term 'chromosome'?
A)
Balbiani
done
clear
B)
Waldeyer
done
clear
C)
Sutton
done
clear
D)
Purkinje
done
clear
View Answer play_arrow
question_answer 320) A chromosome having sub-terminal centromere is called
A)
telocentric
done
clear
B)
acrocentric
done
clear
C)
metacentric
done
clear
D)
sub-metacentric
done
clear
View Answer play_arrow
question_answer 321) How many pairs of contrasting characters were chosen by Mendel for his study with garden pea?
A)
3
done
clear
B)
5
done
clear
C)
7
done
clear
D)
14
done
clear
View Answer play_arrow
question_answer 322) The term 'all elomorphic' implies
A)
any two characters
done
clear
B)
a pair of contrasting characters
done
clear
C)
sex-linked characters
done
clear
D)
a pair of non-contrasting characters
done
clear
View Answer play_arrow
question_answer 323) If a homozygous red flowered plant is crossed with a homozygous white-flowered plant, the offspring will be
A)
all red flowered
done
clear
B)
all white flowered
done
clear
C)
half red flowered
done
clear
D)
half white flowered
done
clear
View Answer play_arrow
question_answer 324) An asexually produced organism inheriting all the characters of its parent is called
A)
clone
done
clear
B)
offspring
done
clear
C)
hybrid
done
clear
D)
variety
done
clear
View Answer play_arrow
question_answer 325) What would be the colour of flowers in \[{{F}_{1}}\] progeny as a result of a cross between homozygous red and homozygous white flowered snapdragon?
A)
Red
done
clear
B)
White
done
clear
C)
Both [a] and
done
clear
D)
[d] Pink
done
clear
View Answer play_arrow
question_answer 326) 26. Who postulated the 'chromosomal theory of \[{{F}_{1}}\]inheritance'?
A)
de Vries
done
clear
B)
Mendel
done
clear
C)
Sutton and Boveri
done
clear
D)
Morgan
done
clear
View Answer play_arrow
question_answer 327) Drosophila melanogaster has
A)
2 pairs of autosomes and 1 pair of us chromosomes
done
clear
B)
3 pairs of autosomes and 1 pair of sex chromosomes
done
clear
C)
1 pair of autosomes and 3 pairs of sex chromosomes
done
clear
D)
2 pairs of autosomes and 2 pairs of sex chromosomes
done
clear
View Answer play_arrow
question_answer 328) The nature of DNA replication is
A)
conservative
done
clear
B)
non-conservative
done
clear
C)
semi-conservative
done
clear
D)
cyanobacteria
done
clear
View Answer play_arrow
question_answer 329) Transformation in bacteria was first discovered by
A)
Lederberg
done
clear
B)
Griffith
done
clear
C)
Fimbriae
done
clear
D)
Tatum
done
clear
View Answer play_arrow
question_answer 330) The drug streptomycin inhibits the process of
A)
prokaryotic translation
done
clear
B)
eukaryotic translation
done
clear
C)
prokaryotic transcription
done
clear
D)
eukaryotic transcription
done
clear
View Answer play_arrow
question_answer 331) The sexuality in bacteria was established by
A)
Lederberg and Tatum
done
clear
B)
H J Muller
done
clear
C)
Hargobind Khorana
done
clear
D)
Ochoa and Kornberg
done
clear
View Answer play_arrow
question_answer 332) The N2 fixing bacterium associated with root nodules of legumes is known as
A)
Azotobacter
done
clear
B)
Nitrobacter
done
clear
C)
Lactobacillus
done
clear
D)
Rhizobium
done
clear
View Answer play_arrow
question_answer 333) The filterable property of Tobacco Mosaic Virus (TMV) was shown by
A)
Ivanowski
done
clear
B)
Beijerinck
done
clear
C)
Stanley
done
clear
D)
Winogradsky
done
clear
View Answer play_arrow
question_answer 334) The bacteria which convert nitrate on molecular nitrogen are called
A)
nitrifying bacteria
done
clear
B)
methanobacteria
done
clear
C)
diazotrophic bacteria
done
clear
D)
denitrifying bacteria
done
clear
View Answer play_arrow
question_answer 335) Endosulphon is a
A)
herbicide
done
clear
B)
weedicide
done
clear
C)
rodenticide
done
clear
D)
pesticide
done
clear
View Answer play_arrow
question_answer 336) The germ theory of disease was propounded by
A)
Koch
done
clear
B)
Pasteur
done
clear
C)
Rayer
done
clear
D)
Devaine
done
clear
View Answer play_arrow
question_answer 337) Interferons are useful in controlling
A)
TB
done
clear
B)
cancer
done
clear
C)
malaria
done
clear
D)
blood pressure
done
clear
View Answer play_arrow
question_answer 338) Yeast is used in the formation of
A)
ammonia
done
clear
B)
alcohol
done
clear
C)
curd
done
clear
D)
petrol
done
clear
View Answer play_arrow
question_answer 339) Fermentation is represented by equation
A)
\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}}\text{+}\,\text{6}{{\text{0}}_{\text{2}}}\to \text{6C}{{\text{O}}_{\text{2}}}\text{+}\,\text{6}{{\text{H}}_{\text{2}}}\text{+}\,\text{673}\,\text{kcal}\]
done
clear
B)
\[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}}\xrightarrow{{}}\text{2}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{OH+}\,\text{2C}{{\text{O}}_{\text{2}}}\text{+}21\,\text{kcal}\]
done
clear
C)
\[6C{{O}_{2}}+12{{H}_{2}}O\to {{\text{C}}_{6}}{{\text{H}}_{12}}{{\text{O}}_{\text{2}}}+{{60}_{2}}\,\]
done
clear
D)
\[\text{6C}{{\text{O}}_{\text{2}}}\text{+6}{{\text{H}}_{\text{2}}}\text{O}\frac{\text{Light}}{\text{Chlorophyll}}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{12}}}{{\text{O}}_{\text{6}}}\text{+6}{{\text{0}}_{\text{2}}}\,\]
done
clear
View Answer play_arrow
question_answer 340) Common bread mould is
A)
yeast
done
clear
B)
Mucor
done
clear
C)
bacteria
done
clear
D)
virus
done
clear
View Answer play_arrow
question_answer 341) Viruses contain
A)
only DNA
done
clear
B)
only RNA
done
clear
C)
either DNA or RNA
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 342) The nitrogen fixation by Nostoc takes place in
A)
vegetative cells
done
clear
B)
akinetes
done
clear
C)
heterocysts
done
clear
D)
hormogonia
done
clear
View Answer play_arrow
question_answer 343) The smallest free-living organism is
A)
virus
done
clear
B)
mycoplasma
done
clear
C)
cyanobacteria
done
clear
D)
bacteria
done
clear
View Answer play_arrow
question_answer 344) The bacterium capable of anaerobic N2 fixation is known as
A)
Clostridium
done
clear
B)
Bacillus
done
clear
C)
Azotobacter
done
clear
D)
Khizobium
done
clear
View Answer play_arrow
question_answer 345) Cyanophages attack
A)
cyanobacteria
done
clear
B)
bacteria
done
clear
C)
fungi
done
clear
D)
lichens
done
clear
View Answer play_arrow
question_answer 346) The bacterial genome contains
A)
DNA and histone
done
clear
B)
DNA or histone
done
clear
C)
DNA without histone
done
clear
D)
Neither DNA nor histone
done
clear
View Answer play_arrow
question_answer 347) Which one of the following statements is correct?
A)
Viruses are obligate parasites
done
clear
B)
All fungi are pathogenic
done
clear
C)
All algae are eukaryotic
done
clear
D)
Bacteria are always harmful to mankind
done
clear
View Answer play_arrow
question_answer 348) The characteristic pigment of cyanobacteria is
A)
fucoxanthin
done
clear
B)
chl-b
done
clear
C)
anthocyanin
done
clear
D)
phycocyanin
done
clear
View Answer play_arrow
question_answer 349) An antiviral chemical produced by the animal cell is
A)
virion
done
clear
B)
interferon
done
clear
C)
represser protein
done
clear
D)
hormone
done
clear
View Answer play_arrow
question_answer 350) The division of the plant kingdom into Prokaryota and Eukaryota is based on the character of
A)
nucleus only
done
clear
B)
chromosomes only
done
clear
C)
cell organelles only
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 351) Who is credited with the introduction of binomial system of nomenclature of plants?
A)
Linnaeus
done
clear
B)
John Ray
done
clear
C)
Bentham and Hooker
done
clear
D)
Aristotle
done
clear
View Answer play_arrow
question_answer 352) Sexual reproduction in Spirogyra is morpho logically characterized by
A)
oogamy
done
clear
B)
anisogamy
done
clear
C)
isogamy
done
clear
D)
Both [a] and [b]
done
clear
View Answer play_arrow
question_answer 353) The moss plants develop from
A)
diploid spores
done
clear
B)
protonema
done
clear
C)
oospores
done
clear
D)
antherozoids
done
clear
View Answer play_arrow
question_answer 354) Zygospores are formed in?
A)
Puccinia
done
clear
B)
Penidllium
done
clear
C)
Altemaria
done
clear
D)
Mucor
done
clear
View Answer play_arrow
question_answer 355) Fern plant is a
A)
haploid gametophyte
done
clear
B)
diploid gametophyte
done
clear
C)
diploid sporophyte
done
clear
D)
haploid sporophyte
done
clear
View Answer play_arrow
question_answer 356) How much time is generally required/taken by the pine plant from pollination to fertilization?
A)
Four months
done
clear
B)
Twelve months
done
clear
C)
Two years
done
clear
D)
Four years
done
clear
View Answer play_arrow
question_answer 357) Pyrenoids are characteristically found in the chloroplast of
A)
fungi
done
clear
B)
algae
done
clear
C)
pteridophytes
done
clear
D)
angiosperms
done
clear
View Answer play_arrow
question_answer 358) Caruncle is formed by
A)
peduncle
done
clear
B)
cotyledons
done
clear
C)
integument
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 359) Perisperm is
A)
outer part of endosperm
done
clear
B)
destroyed synergids
done
clear
C)
destroyed secondary nucleus
done
clear
D)
remains of nucellus
done
clear
View Answer play_arrow
question_answer 360) In pea, the ovule is
A)
hemianatropous
done
clear
B)
amphitropous
done
clear
C)
anatropous
done
clear
D)
campylotropous
done
clear
View Answer play_arrow
question_answer 361) The point where funicle joins with ovule is known as
A)
chalaza
done
clear
B)
hilum
done
clear
C)
integument
done
clear
D)
micropyle
done
clear
View Answer play_arrow
question_answer 362) Aerenchyma is formed in the tissue of
A)
sclerenchyma
done
clear
B)
parenchyma
done
clear
C)
phloem
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 363) Promeristem gives rise to which meristem?
A)
Secondary
done
clear
B)
Lateral
done
clear
C)
Primary
done
clear
D)
Apical
done
clear
View Answer play_arrow
question_answer 364) Whose living cells provide tensile and mechanical strength?
A)
Collenchyma
done
clear
B)
Sclerenchyma
done
clear
C)
Phloem
done
clear
D)
Sclereids
done
clear
View Answer play_arrow
question_answer 365) Which one is not related with plant ash?
A)
Trace elements
done
clear
B)
Essential elements
done
clear
C)
Nitrogen
done
clear
D)
Mineral elements
done
clear
View Answer play_arrow
question_answer 366) 0.1 M solution of a solute has a water potential of
A)
- 2.3 bars
done
clear
B)
zero bar
done
clear
C)
22.4 bars
done
clear
D)
+ 2.3 bars
done
clear
View Answer play_arrow
question_answer 367) A small mesophytic twig with green leaves is dipped into water in a big beaker under sunlight. It demonstrates
A)
photosynthesis
done
clear
B)
respiration
done
clear
C)
transpiration
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 368) Which one is not related to transpiration
A)
Regulation of plant body temperature
done
clear
B)
Absorption and distribution of mineral salts
done
clear
C)
Circulation of water
done
clear
D)
Bleeding
done
clear
View Answer play_arrow
question_answer 369) Which element is essential for photolysis of water?
A)
Nitrogen
done
clear
B)
Chlorine
done
clear
C)
Carbon
done
clear
D)
Oxygen
done
clear
View Answer play_arrow
question_answer 370) In \[{{C}_{4}}\] plants, initial \[C{{O}_{2}}\] fixation takes place in the chloroplasts of
A)
guard cells
done
clear
B)
spongy mesophyll
done
clear
C)
palisade tissue
done
clear
D)
bundle sheath
done
clear
View Answer play_arrow
question_answer 371) Which of the following is formed during respiration?
A)
\[{{O}_{2}}\] (oxygen)
done
clear
B)
\[C{{O}_{2}}\] (carbon dioxide)
done
clear
C)
\[N{{O}_{2}}\] (nitrogen dioxide)
done
clear
D)
\[S{{O}_{2}}\] (sulphur dioxide)
done
clear
View Answer play_arrow
question_answer 372) The pyruvic acid formed in glycolysis is oxidized to \[C{{O}_{2}}\] and \[{{H}_{2}}O\] in a cycle called
A)
Calvin cycle
done
clear
B)
Hill reaction
done
clear
C)
Krebs cycle
done
clear
D)
Nitrogen cycle
done
clear
View Answer play_arrow
question_answer 373) Nitrogenase enzyme is found in Nostoc in the cell of
A)
vegetative
done
clear
B)
heterocyst
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 374) Many of the hydrolytic reactions are
A)
reversible
done
clear
B)
irreversible
done
clear
C)
endothermic
done
clear
D)
exothermic
done
clear
View Answer play_arrow
question_answer 375) Most of the plants are seasonal due to
A)
photoperiodism
done
clear
B)
phototropism
done
clear
C)
photosynthesis
done
clear
D)
photolysis
done
clear
View Answer play_arrow
question_answer 376) Certain chemical sbstances having profound effect on growth are called
A)
catalytic agents
done
clear
B)
phytohormones
done
clear
C)
enzymes
done
clear
D)
compost
done
clear
View Answer play_arrow
question_answer 377) The pyramid of energy in a forest ecosystem is
A)
always upright
done
clear
B)
always inverted
done
clear
C)
Both [a] and [b]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 378) The importance of ecosystem lies in
A)
flow of energy
done
clear
B)
cycling of materials
done
clear
C)
Both [a] and [b]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 379) Which one of the following is a renewable source of energy?
A)
Petroleum
done
clear
B)
Coal
done
clear
C)
Nuclear fuel
done
clear
D)
Trees
done
clear
View Answer play_arrow
question_answer 380) The correct path of energy flow in an ecosystem is
A)
Producers \[\to \]Carnivores\[\to \] Herbivores \[\to \] Decomposers
done
clear
B)
Producers\[\to \]Herbivores \[\to \]Carnivores\[\to \]Decomposers
done
clear
C)
Herbivores \[\to \]Carnivores\[\to \] Producers \[\to \] Decomposers
done
clear
D)
Herbivores \[\to \] Producers \[\to \] Carnivores\[\to \] Decomposers
done
clear
View Answer play_arrow
question_answer 381) The number of primary producers within a specified area would be maximum in
A)
grassland ecosystem
done
clear
B)
forest ecosystem
done
clear
C)
pond ecosystem
done
clear
D)
deserts
done
clear
View Answer play_arrow
question_answer 382) A food chain starts with
A)
nitrogen fixationing organisms
done
clear
B)
photosynthesizing organisms
done
clear
C)
respiration
done
clear
D)
decomposers
done
clear
View Answer play_arrow
question_answer 383) Nepenthes is a
A)
primary producer
done
clear
B)
consumer
done
clear
C)
Both [a] and [b]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 384) Spraying of DDT on crops produces pollution of
A)
air only
done
clear
B)
air and soil only
done
clear
C)
air and water only
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 385) Which of the following is most important source of food?
A)
Lichen
done
clear
B)
Angiosperms
done
clear
C)
Algae
done
clear
D)
Fungi
done
clear
View Answer play_arrow
question_answer 386) Which of the following is a pseudocereal
A)
Triticum aestivum
done
clear
B)
Oryza sativa
done
clear
C)
Zea mays
done
clear
D)
Fagopyrum esculentum
done
clear
View Answer play_arrow
question_answer 387) Which of the following is rich source of protein?
A)
Rice
done
clear
B)
Wheat
done
clear
C)
Groundnut
done
clear
D)
Pearl millet
done
clear
View Answer play_arrow
question_answer 388) Which of the following is surface fibre?
A)
Coir
done
clear
B)
Cotton
done
clear
C)
Sunnhemp
done
clear
D)
Manilahemp
done
clear
View Answer play_arrow
question_answer 389) Which of the following is not a plant fibre?
A)
Cotton
done
clear
B)
Coir
done
clear
C)
Sunnhemp
done
clear
D)
Silk
done
clear
View Answer play_arrow
question_answer 390) Triticum aestivum is
A)
haploid
done
clear
B)
diploid
done
clear
C)
tetraploid
done
clear
D)
hexaploid
done
clear
View Answer play_arrow
question_answer 391) Which of the following is an oilseed crop ?
A)
Careopsis
done
clear
B)
Paddy
done
clear
C)
Sunflower
done
clear
D)
Chrysanthemum
done
clear
View Answer play_arrow
question_answer 392) Richest source of carbohydrate is
A)
Sorghum
done
clear
B)
groundnut
done
clear
C)
gram
done
clear
D)
wheat
done
clear
View Answer play_arrow
question_answer 393) Plants which yield pulses belong to family
A)
Papilionaceae
done
clear
B)
Rosaceae
done
clear
C)
Malvaceae
done
clear
D)
Compositae
done
clear
View Answer play_arrow
question_answer 394) Which state of India is the largest producer of jute?
A)
West Bengal
done
clear
B)
Uttar Pradesh
done
clear
C)
Bihar
done
clear
D)
Madhya Pradesh
done
clear
View Answer play_arrow
question_answer 395) From which part of plant, cotton fibres are obtained?
A)
Root hairs
done
clear
B)
Stem hairs
done
clear
C)
Leaf
done
clear
D)
Seed hairs
done
clear
View Answer play_arrow
question_answer 396) In which part of plant, groundnut oil is stored?
A)
Leaf
done
clear
B)
Tuber
done
clear
C)
Cotyledon
done
clear
D)
Root
done
clear
View Answer play_arrow
question_answer 397) Food can be easily preserved at low temperature because at low temperature
A)
the food can easily be digested
done
clear
B)
the food can easily be cooked
done
clear
C)
the bacterial attack on food is minimized
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 398) Selection is the method of
A)
Plant physiology
done
clear
B)
Plant breeding
done
clear
C)
Genetics
done
clear
D)
Cytology
done
clear
View Answer play_arrow
question_answer 399) Removal of anthers from flowers buds is called
A)
bagging
done
clear
B)
hybridization
done
clear
C)
emasculation
done
clear
D)
heterosis
done
clear
View Answer play_arrow
question_answer 400) What are the main objectives of plant breeding?
A)
More yield
done
clear
B)
Better quality
done
clear
C)
Stress resistance
done
clear
D)
All of these
done
clear
View Answer play_arrow
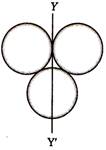
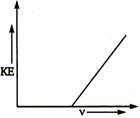
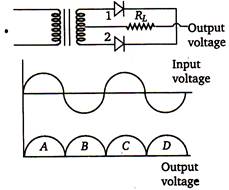 The contribution to output voltage from diode 2 is
The contribution to output voltage from diode 2 is