question_answer 1) The equation of progressive wave is: \[y=0.2\sin 2\pi \left[ \frac{t}{0.01}-\frac{x}{0.3} \right]\]where\[x\]and\[y\]are in metre and t is in second. The velocity of propagation of the wave is:
A)
30 m/s
done
clear
B)
40 m/s
done
clear
C)
300 m/s
done
clear
D)
400 m/s
done
clear
View Answer play_arrow
question_answer 2) A chimpanzee swinging on a swing in a sitting position, stands up suddenly, the time period will:
A)
become infinite
done
clear
B)
remain same
done
clear
C)
increase
done
clear
D)
decrease
done
clear
View Answer play_arrow
question_answer 3) If the ratio of radii of two spheres of same material is 1 : 4 then the ratio of their heat capacities is:
A)
\[\frac{1}{64}\]
done
clear
B)
\[\frac{1}{32}\]
done
clear
C)
\[\frac{1}{16}\]
done
clear
D)
\[\frac{1}{4}\]
done
clear
View Answer play_arrow
question_answer 4) Transverse waves can propagate in:
A)
liquids
done
clear
B)
solids
done
clear
C)
gases
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 5) A box is lying on an inclined plane what is the coefficient of static friction if the box starts sliding when an angle of inclination is\[{{60}^{o}}\]:
A)
\[1.173\]
done
clear
B)
\[~1.732\]
done
clear
C)
\[2.732\]
done
clear
D)
\[~1.677\]
done
clear
View Answer play_arrow
question_answer 6) For which of the following combination of working temperatures, the efficiency of Carnot's engines is highest?
A)
100 K, 80 K
done
clear
B)
80 K, 60 K
done
clear
C)
60 K, 40 K
done
clear
D)
40 K, 20 K
done
clear
View Answer play_arrow
question_answer 7) Myopia is corrected by:
A)
cylindrical lens
done
clear
B)
bifocal lens
done
clear
C)
concave lens
done
clear
D)
convex lens
done
clear
View Answer play_arrow
question_answer 8) On increasing the temperature of a substance gradually, which of the following colours will be noticed by you:
A)
white
done
clear
B)
yellow
done
clear
C)
green
done
clear
D)
red
done
clear
View Answer play_arrow
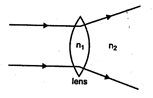
question_answer 9)
The relation between \[{{n}_{1}}\]and \[{{n}_{2}},\]if behaviour of light rays is as shown in figure is:
A)
\[{{n}_{1}}>{{n}_{2}}\]
done
clear
B)
\[{{n}_{2}}>{{n}_{1}}\]
done
clear
C)
\[{{n}_{1}}>{{n}_{2}}\]
done
clear
D)
\[{{n}_{1}}={{n}_{2}}\]
done
clear
View Answer play_arrow
question_answer 10) An aeroplane is flying with a uniform speed of 100 m/s along a circular path of radius 100 m. The angular speed of the aeroplane will be:
A)
1 rad/sec
done
clear
B)
2 rad/sec
done
clear
C)
3 rad/sec
done
clear
D)
4 rad/sec
done
clear
View Answer play_arrow
question_answer 11) In an A.C. circuit the reactance of a coil is \[\sqrt{3}\] times its resistance, the phase difference between the voltage across the coil to the current through the coil will be:
A)
\[\pi /3\]
done
clear
B)
\[\pi /2\]
done
clear
C)
\[\pi /4\]
done
clear
D)
\[\pi /6\]
done
clear
View Answer play_arrow
question_answer 12) \[{{\upsilon }_{1}}\]and\[{{\upsilon }_{2}}\] are the velocities of sound at the same temperature in two monoatomic gases of densities \[{{\rho }_{1}}\]and \[{{\rho }_{2}}\]respectively. If\[{{\rho }_{1}}.{{\rho }_{2}}=\frac{1}{4},\]then the ratio of velocities \[{{\upsilon }_{1}}\]and \[{{\upsilon }_{2}}\]will be:
A)
\[1:2\]
done
clear
B)
\[4:1\]
done
clear
C)
\[2:1\]
done
clear
D)
\[1:4\]
done
clear
View Answer play_arrow
question_answer 13) The resistance of a conductor is 5 ohm at\[50{{\,}^{o}}C\] and 6 ohm at \[100{{\,}^{o}}C.\] Its resistance at \[0{{\,}^{o}}C\]is:
A)
1 ohm
done
clear
B)
2 ohm
done
clear
C)
3 ohm
done
clear
D)
4 ohm
done
clear
View Answer play_arrow
question_answer 14) \[n\]identical droplets charged to the same potential\[V\]coalesce to form a single bigger drop. The potential of the new drop will be:
A)
\[nV\]
done
clear
B)
\[\frac{V}{n}\]
done
clear
C)
\[{{n}^{2/3}}V\]
done
clear
D)
\[n{{V}^{2}}\]
done
clear
View Answer play_arrow
question_answer 15) If in a wire of Young's modulus Y, longitudinal strain X is produced, then the value of potential energy stored in its unit volume will be:
A)
\[0.5\,Y{{X}^{2}}\]
done
clear
B)
\[0.5\,{{Y}^{2}}X\]
done
clear
C)
\[2Y{{X}^{2}}\]
done
clear
D)
\[Y{{X}^{2}}\]
done
clear
View Answer play_arrow
question_answer 16) Light is incident on a glass plate at an angle \[{{60}^{o}},\] the reflected and refracted rays arc mutually perpendicular to each other, then the refractive index of plate will be:
A)
1.732
done
clear
B)
1.5
done
clear
C)
1.4
done
clear
D)
1.5
done
clear
View Answer play_arrow
question_answer 17) A sound source is moving towards a stationary listener with 1/10th of the speed of the sound. The ratio of apparent to real speed will be:
A)
\[{{\left( \frac{11}{10} \right)}^{2}}\]
done
clear
B)
\[{{\left( \frac{9}{10} \right)}^{2}}\]
done
clear
C)
\[\frac{10}{9}\]
done
clear
D)
\[\frac{11}{10}\]
done
clear
View Answer play_arrow
question_answer 18) The Young's double slit experiment, the separation between the slits is halved and the distance between slits and screen is doubled. The fringes width will:
A)
remain the same
done
clear
B)
be quadrupled
done
clear
C)
be doubled
done
clear
D)
be halved
done
clear
View Answer play_arrow
question_answer 19) A source X of unknown frequency produces 8 beats per second, with a source of 250 Hz and 12 beats per second with a source of 270 Hz. Then the frequency of the source X will be:
A)
284 Hz
done
clear
B)
265 Hz
done
clear
C)
258 Hz
done
clear
D)
252 Hz
done
clear
View Answer play_arrow
question_answer 20) The relation between \[\alpha \] and\[\beta \] parameters of a transistor is given by:
A)
\[\alpha =\frac{1-\beta }{\beta }\]
done
clear
B)
\[\frac{\beta }{1-\beta }\]
done
clear
C)
\[\alpha =\frac{1+\beta }{\beta }\]
done
clear
D)
\[\alpha =\frac{\beta }{1+\beta }\]
done
clear
View Answer play_arrow
question_answer 21) A diffraction is obtained by using a beam of red light. What will happen if the red light is replaced by the blue light?
A)
bands will narrower and crowd full together
done
clear
B)
bands become broader and further apart
done
clear
C)
no change will take place
done
clear
D)
bands disappear
done
clear
View Answer play_arrow
question_answer 22) After an interval of one day, 1/6th of the initial amount of a radioactive material remains in a sample. Its half life will be:
A)
2 hour
done
clear
B)
3 hour
done
clear
C)
6 hour
done
clear
D)
12 hour
done
clear
View Answer play_arrow
question_answer 23) Two coherent light beams of intensity I and \[\text{4I}\] are superimposed. The maximum and minimum possible intensities in the resulting beam are:
A)
\[9I\]and \[3I\]
done
clear
B)
\[9I\]and\[I\]
done
clear
C)
\[5I\]and \[3I\]
done
clear
D)
\[5I\]and \[I\]
done
clear
View Answer play_arrow
question_answer 24) If the energy released in the fission of one nucleus is 200 MeV. Then the number of nuclei required per second in a power plant of 16 kW will be:
A)
\[0.5\times {{10}^{14}}\]
done
clear
B)
\[0.5\times {{10}^{12}}\]
done
clear
C)
\[5\times {{10}^{12}}\]
done
clear
D)
\[5\times {{10}^{14}}\]
done
clear
View Answer play_arrow
question_answer 25) Diffraction effects are easier to notice in the case of sound waves than in case of light waves, because the sound waves are:
A)
of longer wavelengths
done
clear
B)
of shorter wavelengths
done
clear
C)
longitudinal waves
done
clear
D)
mechanical waves
done
clear
View Answer play_arrow
question_answer 26) If R is the Rydberg's constant for hydrogen the wave number of the first line in the Lyman series will be:
A)
\[\frac{R}{4}\]
done
clear
B)
\[\frac{3R}{4}\]
done
clear
C)
\[\frac{R}{2}\]
done
clear
D)
\[2R\]
done
clear
View Answer play_arrow
question_answer 27) The resistance of an ideal ammeter will be:
A)
zero
done
clear
B)
small
done
clear
C)
very high
done
clear
D)
infinite
done
clear
View Answer play_arrow
question_answer 28) The energy that should be added to an electron, to reduce its de-Broglie wavelengths from \[{{10}^{-10}}\text{m}\] to\[0.5\times {{10}^{-10}}m,\] will be:
A)
four times the initial energy
done
clear
B)
thrice the initial energy
done
clear
C)
equal to the initial energy
done
clear
D)
twice the initial energy
done
clear
View Answer play_arrow
question_answer 29) A cube of side b has a charge q at each of its vertices. The electric field at the centre of the cube is:
A)
\[\frac{2q}{{{b}^{2}}}\]
done
clear
B)
\[\frac{q}{2{{b}^{2}}}\]
done
clear
C)
zero
done
clear
D)
\[\frac{q}{{{b}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 30) The magnetic flux linked with a coil, in we bers, is given by the equations \[\text{o }\!\!|\!\!\text{ = 3}{{\text{t}}^{2}}+4t+9.\] Then the magnitude of induced emf at \[t=2\]second will be
A)
2 volt
done
clear
B)
4 volt
done
clear
C)
8 volt
done
clear
D)
16 volt
done
clear
View Answer play_arrow
question_answer 31) A hot wire ammeter reads 10 amp. In an A.C. circuit. The peak value of the current will be:
A)
\[\frac{3}{\pi }\text{amp}\]
done
clear
B)
\[\frac{10}{\sqrt{2}}\text{amp}\]
done
clear
C)
\[10\sqrt{2}\,\text{amp}\]
done
clear
D)
\[6\pi \,\text{amp}\]
done
clear
View Answer play_arrow
question_answer 32)
A and B are two concentric circular conductors of centre O and carrying
A)
\[\frac{1}{2}\]
done
clear
B)
\[\frac{1}{3}\]
done
clear
C)
\[\frac{1}{4}\]
done
clear
D)
\[\frac{1}{6}\]
done
clear
View Answer play_arrow
question_answer 33) Two capacitors of capacitance \[{{C}_{1}}\]and \[{{C}_{2}}\]are charged to potentials \[{{V}_{1}}\]and\[{{V}_{2}}\] respectively. When they are connected in parallel the ratio of their respective charges will be:
A)
\[\frac{{{C}_{1}}}{{{C}_{2}}}\]
done
clear
B)
\[\frac{C_{1}^{2}}{C_{2}^{2}}\]
done
clear
C)
\[\frac{{{V}_{1}}}{{{V}_{2}}}\]
done
clear
D)
\[\frac{V_{1}^{2}}{V_{2}^{2}}\]
done
clear
View Answer play_arrow
question_answer 34) An example of a diamagnetic substance is:
A)
aluminium
done
clear
B)
copper
done
clear
C)
iron
done
clear
D)
nickel
done
clear
View Answer play_arrow
question_answer 35) In a charged capacitor, the energy is stored in:
A)
centre of the plates
done
clear
B)
edges of the plates
done
clear
C)
between the plates
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 36) Relative permeability of iron is 5500, then its magnetic susceptibility will be:
A)
\[5500\times {{10}^{7}}\]
done
clear
B)
\[5500\times {{10}^{-7}}\]
done
clear
C)
\[5501\]
done
clear
D)
\[5499\]
done
clear
View Answer play_arrow
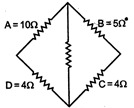
question_answer 37)
In a typical Wheatstone network the resistances in cyclic order are \[A=10\,\Omega ,B=5\Omega ,\,C=4\,\Omega \] and\[D=4\Omega \]for the bridge to be balanced:
A)
\[10\,\Omega \]should be connected in parallel with A
done
clear
B)
\[10\,\Omega \] should be connected in series with A
done
clear
C)
\[5\,\Omega \] should be connected in series with B
done
clear
D)
\[5\,\Omega \] should be connected in parallel with B
done
clear
View Answer play_arrow
question_answer 38) An electron of mass m and charge q is travelling with a speed v along a circular path of radius r at right angles to a uniform magnetic field B, if speed of the electron is doubled and magnetic field is halved, then the resulting path would have a radius:
A)
\[4r\]
done
clear
B)
\[2r\]
done
clear
C)
\[\frac{r}{2}\]
done
clear
D)
\[\frac{r}{4}\]
done
clear
View Answer play_arrow
question_answer 39) Two resistances \[{{r}_{1}}\]and\[{{r}_{2}}({{r}_{1}}<{{r}_{2}})\]are connected in parallel. The equivalent resistance R is such that:
A)
\[R>({{r}_{1}}+{{r}_{2}})\]
done
clear
B)
\[{{r}_{2}}<R<({{r}_{1}}+{{r}_{2}})\]
done
clear
C)
\[{{r}_{1}}<R<{{r}_{2}}\]
done
clear
D)
\[R<{{r}_{1}}\]
done
clear
View Answer play_arrow
question_answer 40) The potential at a point, due to a positive charge of \[\text{100}\,\text{ }\!\!\mu\!\!\text{ C}\]at a distance of 9 m, is:
A)
\[{{10}^{7}}\,\text{volt}\]
done
clear
B)
\[{{10}^{6}}\,\text{volt}\]
done
clear
C)
\[{{10}^{5}}\,\text{volt}\]
done
clear
D)
\[{{10}^{4}}\,\text{volt}\]
done
clear
View Answer play_arrow
question_answer 41) Four wires of identical length, diameters and of the same material are stretched on a sonometre wire. If the ratio of their tensions is 1 : 4 : 9 : 16 then the ratio of their fundamental frequencies are:
A)
16 : 9 : 4 : 1
done
clear
B)
4 : 3 : 2 : 1
done
clear
C)
1 : 4 : 2 : 16
done
clear
D)
1 : 2 : 3 : 4
done
clear
View Answer play_arrow
question_answer 42) An echo is heard when the minimum distance of the reflecting surface is:
A)
47 m
done
clear
B)
34 m
done
clear
C)
17 m
done
clear
D)
9 m
done
clear
View Answer play_arrow
question_answer 43) In which process the P-V indicator diagram is a straight line parallel to volume axis?
A)
irreversible
done
clear
B)
adiabatic
done
clear
C)
isothermal
done
clear
D)
isobaric
done
clear
View Answer play_arrow
question_answer 44) The kinetic energy of a body becomes four times its initial value. Then the linear momentum will be:
A)
four times the initial value
done
clear
B)
three times the initial value
done
clear
C)
twice the initial value
done
clear
D)
same as the initial value
done
clear
View Answer play_arrow
question_answer 45) At which temperature, the Fahrenheit and Centigrade scales are equal:
A)
\[-{{48}^{o}}\]
done
clear
B)
\[-{{40}^{o}}\]
done
clear
C)
\[{{37}^{o}}\]
done
clear
D)
\[{{40}^{o}}\]
done
clear
View Answer play_arrow
question_answer 46) A uniform wire of resistance R is uniformly compressed along its length, until the radius becomes\[n\]times the original radius. Now resistance of the wire becomes:
A)
\[nR\]
done
clear
B)
\[\frac{R}{n}\]
done
clear
C)
\[\frac{R}{{{n}^{2}}}\]
done
clear
D)
\[\frac{R}{{{n}^{4}}}\]
done
clear
View Answer play_arrow
question_answer 47) The impurity atom that should be added the germanium to make it \[n-\]type will be:
A)
aluminium
done
clear
B)
arsenic
done
clear
C)
indium
done
clear
D)
iodine
done
clear
View Answer play_arrow
question_answer 48) The waves that can not be polarized are:
A)
electromagnetic waves
done
clear
B)
longitudinal waves
done
clear
C)
transverse waves
done
clear
D)
light waves
done
clear
View Answer play_arrow
question_answer 49) An empty vessel is partially filled with water, then the frequency of vibration of air column in the vessel:
A)
remains same
done
clear
B)
decreases
done
clear
C)
increases
done
clear
D)
first increases then decreases
done
clear
View Answer play_arrow
question_answer 50) A man suffering from short sight is unable to see objects distinctly at a distance more than 2m. The power of lens required to correct this defect of the eye should be:
A)
\[~+\,2D\]
done
clear
B)
\[~+\text{ }0.50\,D\]
done
clear
C)
\[-2D\]
done
clear
D)
\[-0.5D\]
done
clear
View Answer play_arrow
question_answer 51) A monoatomic gas is suddenly compressed to l/8th of its initial volume adiabatically. The ratio of its final pressure to initial pressure will be \[(\gamma =5/3):\]
A)
8
done
clear
B)
24
done
clear
C)
32
done
clear
D)
40
done
clear
View Answer play_arrow
question_answer 52) 2 kg mass is rotating on a circular path of radius 0.8 m with an angular velocity of 44 rad/sec. If the radius of the path becomes 1 m. Then value of angular velocity is:
A)
\[35.32\,\text{rad/sec}\]
done
clear
B)
\[28.16\,\text{rad/sec}\]
done
clear
C)
\[14-08\,\text{rad/sec}\]
done
clear
D)
\[7\,\text{rad/sec}\]
done
clear
View Answer play_arrow
question_answer 53) A whistle of frequency 500 Hz tied to the end of a string of length 1-2 m revolves at 400 rev/min. A listener standing some distance away in the plane of rotation of whistle nears frequencies in the rays (speed of sound =340 m/s) :
A)
436 to 586
done
clear
B)
426 to 574
done
clear
C)
436 to 574
done
clear
D)
436 to 574
done
clear
View Answer play_arrow
question_answer 54) The dispersive powers of crown and flint glasses are 0-02 and 0-04 respectively. In an achromatic combination of lenses the focal length of flint glass lens is 40 cm. The focal length of crown glass lens will be:
A)
- 20 cm
done
clear
B)
+ 20 cm
done
clear
C)
-10 cm
done
clear
D)
+ 20 cm
done
clear
View Answer play_arrow
question_answer 55) Two insulated metallic spheres of capacitances \[\text{3 }\!\!\mu\!\!\text{ F}\]and \[\text{5 }\!\!\mu\!\!\text{ F}\]are charged to 300 volts and 500 volts respectively. The energy loss when they are connected by a wire is:
A)
\[0-035\text{ J}\]
done
clear
B)
\[1-5\,\text{J}\]
done
clear
C)
\[3-75\,\text{J}\]
done
clear
D)
\[\text{ }\!\!~\!\!\text{ 0}\text{.0375 J}\]
done
clear
View Answer play_arrow
question_answer 56) The gardner waters the plants by a pipe of diameter 1 mm. The water comes out at the rate of \[\text{10}\,\text{c}{{\text{m}}^{\text{3}}}\text{/sec}\]. The reactionary force exerted on the hand of the gardner is:
A)
zero
done
clear
B)
\[1.27\times {{10}^{-2}}N\]
done
clear
C)
\[1.27\times {{10}^{-4}}N\]
done
clear
D)
\[0.127\,N\]
done
clear
View Answer play_arrow
question_answer 57) A thin hollow cylinder open at both ends: (i) sliding without rotating (ii) rolls without slipping, with the same speed the ratio of kinetic energy in the two cases is:
A)
1:1
done
clear
B)
4:1
done
clear
C)
1:2
done
clear
D)
2:1
done
clear
View Answer play_arrow
question_answer 58) A rain drop of radius 0.3 mm has a terminal velocity of 1 m/s in air. The viscosity of air is \[18\times {{10}^{-5}}\]poise. The visious forces on the drop will be:
A)
\[16.95\times {{10}^{-9}}N\]
done
clear
B)
\[1.695\times {{10}^{-9}}N\]
done
clear
C)
\[10.17\times {{10}^{-9}}N\]
done
clear
D)
\[101.73\times {{10}^{-9}}N\]
done
clear
View Answer play_arrow
question_answer 59) The force required to separate two glass plates of area \[{{10}^{-2}}{{\text{m}}^{\text{2}}}\]with a film of water 0.05 mm thick between them, is (Surface tension of water is \[70\times {{10}^{-3}}N/m\])
A)
28 N
done
clear
B)
14 N
done
clear
C)
50 N
done
clear
D)
38 N
done
clear
View Answer play_arrow
question_answer 60) The dimension of\[\frac{1}{2}{{\varepsilon }_{0}}{{E}^{2}}\](\[{{\varepsilon }_{0}}\]is the permittivity of free space, and E is electric field), is:
A)
\[[M{{L}^{2}}{{T}^{-1}}]\]
done
clear
B)
\[[M{{L}^{-1}}{{T}^{-2}}]\]
done
clear
C)
\[[M{{L}^{2}}{{T}^{-2}}]\]
done
clear
D)
\[[ML{{T}^{-1}}]\]
done
clear
View Answer play_arrow
question_answer 61) Which one of the following defects in the crystals lowers its density?
A)
F centres
done
clear
B)
Interstitial defect
done
clear
C)
Frenkel defect
done
clear
D)
Schottky
done
clear
View Answer play_arrow
question_answer 62) \[{{H}_{2}}(g)+C{{l}_{2}}(g)\to 2HCl(g).\] \[\Delta H=-44k.cal\] \[2Na(s)+2HCl(g)\to 2NaCl(s)+{{H}_{2}}(g).\] \[\Delta H=-152\,\text{k}\text{.cal}\] For the reaction, \[Na(s)+\frac{1}{2}C{{l}_{2}}(g)\to NaCl(s),\Delta H =?\]
A)
\[-108\,k.cal\]
done
clear
B)
\[-196\,k.cal\]
done
clear
C)
\[-98\,k.cal\]
done
clear
D)
\[54\,k.cal\]
done
clear
View Answer play_arrow
question_answer 63) The fatty acid that shows reducing property is:
A)
acetic acid
done
clear
B)
ethanoic acid
done
clear
C)
oxalic acid
done
clear
D)
formic acid
done
clear
View Answer play_arrow
question_answer 64) By adding =\[\text{20}\,\text{ml}\,\text{0}\text{.1}\,\text{N}\,\text{HCl}\]to 20 ml \[\text{0}\text{.001}\,\text{N}\,\text{KOH,}\]the pH of the obtained solution will be:
A)
2
done
clear
B)
1.3
done
clear
C)
0
done
clear
D)
7
done
clear
View Answer play_arrow
question_answer 65) In the synthesis of ammonia \[{{N}_{2}}(g)+3{{H}_{2}}\rightleftharpoons 2N{{H}_{3}}(g)\] When 100 ml. of \[{{\text{N}}_{\text{2}}}\] has reacted, the volume of \[{{H}_{2}}\] which has also reacted and ammonia produced are:
A)
\[\text{300 ml}\text{. }{{\text{H}}_{\text{2 }}}\]and \[\text{200 ml}\text{. N}{{\text{H}}_{\text{3}}}\]
done
clear
B)
\[\text{ }\!\!~\!\!\text{ 300 ml}\text{. }{{\text{H}}_{\text{2}}}\] and \[\text{300 ml}\text{. N}{{\text{H}}_{\text{3}}}\]
done
clear
C)
\[\text{100 ml}\text{. }{{\text{H}}_{\text{2}}}\]and \[\text{100 ml}\text{. N}{{\text{H}}_{\text{3}}}\]
done
clear
D)
\[\text{100 ml}\text{. }{{\text{H}}_{\text{2}}}\]and \[\text{200 ml}\text{. N}{{\text{H}}_{\text{3}}}\]
done
clear
View Answer play_arrow
question_answer 66) When 9-65 coulombs of electricity is passed through a solution of silver nitrate (atomic mass of\[Ag=108\,g\,mo{{l}^{-1}}\]), the amount of silver deposited is:
A)
16 - 2 mg
done
clear
B)
21 - 2 mg
done
clear
C)
10 - 8 mg
done
clear
D)
6 - 4 mg
done
clear
View Answer play_arrow
question_answer 67) The strongest Bronsted base in the following anion is:
A)
\[ClO_{3}^{-}\]
done
clear
B)
\[ClO_{4}^{-}\]
done
clear
C)
\[Cl{{O}^{-}}\]
done
clear
D)
\[ClO_{2}^{-}\]
done
clear
View Answer play_arrow
question_answer 68) Bleaching powder loses its power on keeping for a long time because:
A)
it changes into calcium hypochlorate
done
clear
B)
it changes into calcium chloride and calcium hydroxide
done
clear
C)
it absorbs moisture
done
clear
D)
it changes into calcium chloride and calcium chlorate
done
clear
View Answer play_arrow
question_answer 69) When potassium ferrocyanide crystals are heated with concentrated sulphuric acid, the gas evolved is:
A)
sulphur dioxide
done
clear
B)
ammonia
done
clear
C)
carbon dioxide
done
clear
D)
carbon monoxide
done
clear
View Answer play_arrow
question_answer 70) \[{{H}_{2}}+C{{l}_{2}}\xrightarrow{{}}2HCl,\Delta H=194\,kJ.\]In this reaction heat of formation of HCl in kJ is:
A)
\[+914\,kJ\]
done
clear
B)
\[+\,97\,kJ\]
done
clear
C)
\[-\,97\,kJ\]
done
clear
D)
\[-194\,kJ\]
done
clear
View Answer play_arrow
question_answer 71) One mole of an ideal gas is allowed to expand reversibly and adiabatically from a temperature of \[\text{27}{{\,}^{\text{o}}}\text{C}\text{.}\] If the work done during the process is 3 kJ, the final temperature will be equal to\[\text{(C}\upsilon \text{=20}\,\text{J}\,{{\text{K}}^{-1}}\text{)}\]
A)
\[~150\text{ }K\]
done
clear
B)
\[~100\text{ }K\]
done
clear
C)
\[26.85{{\,}^{o}}C\]
done
clear
D)
\[~295K\]
done
clear
View Answer play_arrow
question_answer 72) Solution of sodium metal in liquid ammonia is strongly reducing due to the presence in the solution of the following:
A)
sodium hydride
done
clear
B)
odium amide
done
clear
C)
sodium atoms
done
clear
D)
solvated electrons
done
clear
View Answer play_arrow
question_answer 73) The IUPAC name of\[C{{H}_{3}}-C{{H}_{2}}-\underset{C{{H}_{3}}}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{C{{H}_{3}}}{\overset{{{C}_{4}}{{H}_{9}}}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-C{{H}_{3}}\]is:
A)
3, 4, 4-trimethyl-heptane
done
clear
B)
3, 4, 4-trimethyl-octane
done
clear
C)
2-butyl, 2-methyl, 3 ethyl butane
done
clear
D)
2-ethyl, 3, 3-dimethyl-heptane
done
clear
View Answer play_arrow
question_answer 74) Which of the following has lowest percentage of carbon?
A)
steel
done
clear
B)
all have same percentage
done
clear
C)
cast ion
done
clear
D)
wrought iron
done
clear
View Answer play_arrow
question_answer 75) When ethanal is heated with Fehling's solution it gives a precipitate of:
A)
\[\text{CuO}\]
done
clear
B)
\[\text{C}{{\text{u}}_{\text{2}}}\text{O}\,\text{+}\,\text{CuO}\]
done
clear
C)
\[\text{Cu}\]
done
clear
D)
\[C{{u}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 76) The number of unpaired electrons in\[F{{e}^{3+}}(Z=26)\] are:
A)
5
done
clear
B)
6
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 77) The\[pKa\]of a certain weak acid is 4-0. What should be the [salt] to [acid] ratio if we have to prepare a buffer with pH=5 using the acid and one of the salts?
A)
10:1
done
clear
B)
1:10
done
clear
C)
4:5
done
clear
D)
5:4
done
clear
View Answer play_arrow
question_answer 78) Which one of the following contains both covalent and ionic bonds?
A)
\[N{{H}_{4}}Cl\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[CC{{l}_{4}}\]
done
clear
D)
\[CaC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 79) The nitride ion in lithium nitride is composed of:
A)
7 protons + 10 electrons
done
clear
B)
10 protons + 10 electrons
done
clear
C)
7 protons + 7 protons
done
clear
D)
10 protons + 7 electrons
done
clear
View Answer play_arrow
question_answer 80) In a face centred cubic cell, an atom at the face contributes to the unit cell:
A)
1/4 part
done
clear
B)
1/8 part
done
clear
C)
1 part
done
clear
D)
1/2 part
done
clear
View Answer play_arrow
question_answer 81) Unpleasant smelling carbylamines are formed by heating alkali and chloroform with:
A)
any aliphatic amine
done
clear
B)
any aromatic amine
done
clear
C)
any amine
done
clear
D)
any primary amine
done
clear
View Answer play_arrow
question_answer 82) Which of the following alkyl halides is used as a methylating agent?
A)
\[{{C}_{2}}{{H}_{5}}Br\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}Cl\]
done
clear
C)
\[C{{H}_{3}}Cl\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}Cl\]
done
clear
View Answer play_arrow
question_answer 83) A radioactive isotope having a half life of 3 days was received after 12 days. It was found that there were 3 g of the isotope in the container. The initial weight of the isotope when packed was:
A)
36 g
done
clear
B)
48 g
done
clear
C)
12 g
done
clear
D)
24 g
done
clear
View Answer play_arrow
question_answer 84) Cuprous ion is colourless while cupric ion is coloured because:
A)
both have half filled p and d-orbitals
done
clear
B)
cuprous ion has incomplete d-orbital and cupric ion has a complete \[d-\]orbital
done
clear
C)
both have unpaired electrons in the d-orbitals
done
clear
D)
cuprous ion has a complete d-orbital and cupric ion has an incomplete \[d-\]orbital
done
clear
View Answer play_arrow
question_answer 85) Which of the following ions can cause coagulation of proteins?
A)
\[A{{g}^{+}}\]
done
clear
B)
\[N{{a}^{+}}\]
done
clear
C)
\[M{{g}^{+\,+}}\]
done
clear
D)
\[C{{a}^{+\,+}}\]
done
clear
View Answer play_arrow
question_answer 86) Number of molecules in one litre of water is close to:
A)
\[\frac{18}{22.4}\times {{10}^{23}}\]
done
clear
B)
\[55.5\times 6.023\times {{10}^{23}}\]
done
clear
C)
\[\frac{6.023}{23.4}\times {{10}^{23}}\]
done
clear
D)
\[18\times 6.023\times {{10}^{23}}\]
done
clear
View Answer play_arrow
question_answer 87) Which of the following alkali metal ions has lowest ionic mobility in aqueous solution?
A)
\[R{{b}^{+}}\]
done
clear
B)
\[C{{s}^{+}}\]
done
clear
C)
\[Li\]
done
clear
D)
\[N{{a}^{+}}\]
done
clear
View Answer play_arrow
question_answer 88) At \[\text{490}{{\,}^{o}}\text{C,}\] the equilibrium constant for the synthesis of \[\text{HI}\] is 50, the value of K for the dissociation of HI will be:
A)
\[\text{20}\text{.0}\]
done
clear
B)
\[2.0\]
done
clear
C)
\[0.2\]
done
clear
D)
\[0.02\]
done
clear
View Answer play_arrow
question_answer 89) For which of the following reactions are the numerical value of \[{{K}_{p}}\]and \[{{K}_{c}}\]the same?
A)
\[{{H}_{2}}(g)+C{{l}_{2}}(g)\rightleftharpoons 2HCl(g)\]
done
clear
B)
\[{{H}_{2}}(g)+{{I}_{2}}(g)\rightleftharpoons 2HI(g)\]
done
clear
C)
\[2NOCl(g)\rightleftharpoons 2NO(g)+C{{l}_{2}}(g)\]
done
clear
D)
\[{{N}_{2}}(g)+3{{H}_{2}}(g)\rightleftharpoons 2N{{H}_{3}}(g)\]
done
clear
View Answer play_arrow
question_answer 90) The number of \[\pi -\]bonds in\[C{{H}_{2}}=CH-CH=CH-C\equiv CH\]is:
A)
2
done
clear
B)
5
done
clear
C)
4
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 91) The normality of \[2.3\,\text{M}\,{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]solution is:
A)
\[2.3\,N\]
done
clear
B)
\[4.6\,N\]
done
clear
C)
\[0.46\,N\]
done
clear
D)
\[0.23\,N\]
done
clear
View Answer play_arrow
question_answer 92) Hydrolytic reaction of fats with caustic soda is known as:
A)
acetylation
done
clear
B)
carboxylation
done
clear
C)
esterification
done
clear
D)
saponification
done
clear
View Answer play_arrow
question_answer 93) Aluminium chloride is a/an:
A)
Bronsted Lowry acid
done
clear
B)
Arrhenius acid
done
clear
C)
Lewis acid
done
clear
D)
Lewis acid
done
clear
View Answer play_arrow
question_answer 94) A reaction is \[A+B\rightleftharpoons C+D\]initially. We start with equal concentration of A arid B. At equilibrium we find the moles of C is two times that of A. What is the equilibrium constant of the reaction?
A)
1/4
done
clear
B)
½
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 95) Which of the following is not used in Friedel-Crafts reaction?
A)
phenyl acetanilide
done
clear
B)
bromobenzene
done
clear
C)
benzene
done
clear
D)
chlorobenzene
done
clear
View Answer play_arrow
question_answer 96) On heating sodium metal in current of dry ammonia, the compound formed is:
A)
sodium amide
done
clear
B)
sodium azide
done
clear
C)
sodium nitride
done
clear
D)
sodium hydride
done
clear
View Answer play_arrow
question_answer 97) Hydrogen bonding is maximum in:
A)
ethyl chloride
done
clear
B)
triethyl amine
done
clear
C)
ethanol
done
clear
D)
diethyl ether
done
clear
View Answer play_arrow
question_answer 98) Phenol is heated with pthalic anhydride in presence of cone. \[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{.}\]The product gives pink colour with alkali. The product is:
A)
phenolphthalein
done
clear
B)
bakelite
done
clear
C)
salicylic acid
done
clear
D)
fluorescein
done
clear
View Answer play_arrow
question_answer 99) A reaction that is of the first order with respect to reactant A has a rate constant \[6\,{{\min }^{-1}}.\]If we start with \[[A]=\text{0}\text{.5}\,\text{mol}\,{{\text{L}}^{-1}},\] when would [A] reach the value \[0.05\,\text{mol}\,{{\text{L}}^{-1}}?\]
A)
\[\text{0}\text{.384 min}\]
done
clear
B)
\[\text{0}\text{.15}\,\text{min}\]
done
clear
C)
3 min
done
clear
D)
\[\text{3}\text{.84}\,\text{min}\]
done
clear
View Answer play_arrow
question_answer 100) Helium atom is two times heavier than a hydrogen molecule at 298 K. The average kinetic energy of helium is:
A)
four times that of a hydrogen molecule
done
clear
B)
half that of a hydrogen molecule
done
clear
C)
two times that of a hydrogen molecule
done
clear
D)
same as that of a hydrogen molecule
done
clear
View Answer play_arrow
question_answer 101) Froth floatation process is used for the metallurgy of:
A)
chloride ores
done
clear
B)
amalgams
done
clear
C)
oxide ores
done
clear
D)
sulphide ores
done
clear
View Answer play_arrow
question_answer 102) 10 litre solution of urea contains 240 g urea. The active mass of urea will be:
A)
\[0.04\]
done
clear
B)
\[0.02\]
done
clear
C)
\[0.4\]
done
clear
D)
\[0.2\]
done
clear
View Answer play_arrow
question_answer 103) Which of the following does not reduce Benedict's solution?
A)
sucrose
done
clear
B)
aldehyde
done
clear
C)
glucose
done
clear
D)
fructose
done
clear
View Answer play_arrow
question_answer 104) Matte contains mainly:
A)
\[\text{C}{{\text{u}}_{\text{2}}}\text{S}\]and \[\text{FeS}\]
done
clear
B)
\[\text{CuS}\]and \[\text{F}{{\text{e}}_{\text{2}}}{{\text{S}}_{\text{3}}}\]
done
clear
C)
\[\text{Fe}\]
done
clear
D)
\[\text{C}{{\text{u}}_{\text{2}}}\text{S}\]
done
clear
View Answer play_arrow
question_answer 105) The name of the product obtained by the addition of\[\text{HI}\]to propene in presence of peroxide catalyst is:
A)
isopropyl iodide
done
clear
B)
2-iodo propene
done
clear
C)
2-iodopropane
done
clear
D)
1-iodopropane
done
clear
View Answer play_arrow
question_answer 106) In Nessler's reagent the ion present is:
A)
\[\text{Hg}{{\text{I}}^{2-}}\]
done
clear
B)
\[\text{HgI}_{4}^{2-}\]
done
clear
C)
\[H{{g}^{+}}\]
done
clear
D)
\[\text{H}{{\text{g}}^{\text{+}\,\text{+}}}\]
done
clear
View Answer play_arrow
question_answer 107) The calorific value is maximum in case of:
A)
milk
done
clear
B)
minerals
done
clear
C)
carbohydrates
done
clear
D)
proteins
done
clear
View Answer play_arrow
question_answer 108) Flux is used to:
A)
remove all impurities from ores
done
clear
B)
reduce metal oxide
done
clear
C)
remove silica
done
clear
D)
remove silica and undesirable metal oxides
done
clear
View Answer play_arrow
question_answer 109) Which of the following molecules has the largest root mean square velocity at \[\text{25}{{\,}^{\text{o}}}\text{C?}\]
A)
\[\text{C}{{\text{O}}_{\text{2}}}\]
done
clear
B)
\[\text{S}{{\text{O}}_{\text{2}}}\]
done
clear
C)
\[{{H}_{2}}S\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 110) Stainless steel contains:
A)
\[Fe+Cr+Cu\]
done
clear
B)
\[Fe+C+Ni\]
done
clear
C)
\[Fe+Cr+Ni\]
done
clear
D)
\[Fe+Ni+Cu\]
done
clear
View Answer play_arrow
question_answer 111) The limiting radius ratio for tetrahedral shape is:
A)
0.225 to 0.414
done
clear
B)
0.414 to 0.732
done
clear
C)
0 to 0.155
done
clear
D)
0.155 to 0.225
done
clear
View Answer play_arrow
question_answer 112) For the process dry \[\text{ice}\xrightarrow{{}}\text{C}{{\text{O}}_{\text{2}}}\text{(g)}\]
A)
\[\Delta H\]is positive while \[\Delta \rho \]is negative
done
clear
B)
both\[\Delta H\] and are negative
done
clear
C)
both \[\Delta H\]and \[\Delta \rho \]are positive
done
clear
D)
\[\Delta H\]is negative while \[\Delta \rho \]is positive
done
clear
View Answer play_arrow
question_answer 113) \[N{{H}_{3}}\]and \[B{{F}_{3}}\]form an adduct readily because they form:
A)
a co-ordinate bond
done
clear
B)
a hydrogen bond
done
clear
C)
an ionic bond
done
clear
D)
a covalent bond
done
clear
View Answer play_arrow
question_answer 114) The brown ring test for \[\text{N}{{\text{O}}_{\text{2}}}\]and \[\text{NO}_{3}^{-}\]is due to the formation of complex ion with the formula:
A)
\[{{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
B)
\[{{[Fe(NO){{(CN)}_{5}}]}^{2+}}\]
done
clear
C)
\[{{[Fe{{({{H}_{2}}O)}_{5}}NO]}^{2+}}\]
done
clear
D)
\[{{[Fe({{H}_{2}}O){{(NO)}_{5}}]}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 115) Amino acids usually exist in the from of Zwitter ions. This means that it consists of:
A)
the basic group \[-\text{N}{{\text{H}}_{\text{2}}}\]and the acidic group\[-\text{COOH}\]
done
clear
B)
the basic group\[-\text{NH}_{\text{3}}^{\text{+}}\]and the acidic group\[-\text{C}{{\text{O}}_{\text{2}}}\]
done
clear
C)
the basic group \[-\text{CO}_{2}^{-}\]and the acidic group\[-\text{NH}_{\text{3}}^{\text{+}}\]
done
clear
D)
no acidic or basic group
done
clear
View Answer play_arrow
question_answer 116) A certain compound gives negative test with ninhydrin and positive test with Benedict's solution; it is:
A)
an ammo acid
done
clear
B)
a monosaccharide
done
clear
C)
a lipid
done
clear
D)
a protein
done
clear
View Answer play_arrow
question_answer 117) If formaldehyde and KOH are heated we get:
A)
methane
done
clear
B)
methyl alcohol
done
clear
C)
ethyl for mate
done
clear
D)
acetylene
done
clear
View Answer play_arrow
question_answer 118) Which of the following reagents is used to distinguish ethene from ethyne?
A)
bromine in \[CC{{l}_{4}}\]
done
clear
B)
ammonical \[C{{u}_{2}}C{{l}_{2}}\]
done
clear
C)
bromine water
done
clear
D)
alkaline \[KMn{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 119) Brass is an alloy of:
A)
\[Al\]and \[Zn\]
done
clear
B)
\[Cu\]and\[Al\]
done
clear
C)
\[Ni\]and \[Zn\]
done
clear
D)
\[Cu\]and \[Zn\]
done
clear
View Answer play_arrow
question_answer 120)
60.
A)
\[SnC{{l}_{2}}\]
done
clear
B)
\[NH_{3}^{+}C{{l}^{-}}\]
done
clear
C)
\[N{{H}_{2}}\]
done
clear
D)
\[Cl\]
done
clear
View Answer play_arrow
question_answer 121) If \[a=\cos \theta +i\sin \theta ,\]then \[\frac{1+a}{1-a}=\]
A)
\[\cot \theta \]
done
clear
B)
\[\cot \theta /2\]
done
clear
C)
\[i\cot \theta /2\]
done
clear
D)
\[i\tan \theta /2\]
done
clear
View Answer play_arrow
question_answer 122) General solution of tan \[5\theta =\cot 2\theta \] is:
A)
\[\theta =\frac{n\pi }{7}+\frac{\pi }{14}\]
done
clear
B)
\[\theta =\frac{n\pi }{7}+\frac{\pi }{5}\]
done
clear
C)
\[\theta =\frac{n\pi }{7}+\frac{\pi }{2}\]
done
clear
D)
\[\theta =\frac{n\pi }{7}+\frac{\pi }{3},n\in Z\]
done
clear
View Answer play_arrow
question_answer 123) If \[\sin y=x\sin (a+y),\]then\[\frac{dy}{dx}=\]
A)
\[\frac{\sin \sqrt{a}}{\sin (a+y)}\]
done
clear
B)
\[\frac{{{\sin }^{2}}(a+y)}{\sin a}\]
done
clear
C)
\[\frac{\sin (a+y)}{\sin a}\]
done
clear
D)
\[\frac{\cos (a+y)}{\cos a}\]
done
clear
View Answer play_arrow
question_answer 124) If \[x=\frac{1-{{t}^{2}}}{1+{{t}^{2}}}\]and \[y=\frac{2t}{1+{{t}^{2}}}\]then \[\frac{dy}{dx}\]
A)
\[-y/x\]
done
clear
B)
\[y/x\]
done
clear
C)
\[-x/y\]
done
clear
D)
\[x/y\]
done
clear
View Answer play_arrow
question_answer 125) If \[{{2}^{x}}+{{2}^{y}}={{2}^{x+y}},\]then the value of \[\frac{dy}{dx}\]at \[x=y=1\]is:
A)
zero
done
clear
B)
\[-1\]
done
clear
C)
1
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 126) The angle of elevation of the top of a tower A from the top B and bottom D at a building of height a are \[{{30}^{o}}\] and\[{{45}^{o}}\]respectively. If the tower and the building stand at the same level, then the height of the tower is:
A)
\[a\sqrt{3}\]
done
clear
B)
\[\frac{a\sqrt{3}}{\sqrt{3}-1}\]
done
clear
C)
\[\frac{a(3+\sqrt{3})}{2}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 127) If \[\log x256=8/5,\]then \[x\]is equal to:
A)
64
done
clear
B)
16
done
clear
C)
32
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 128) If \[\log 2,\log ({{2}^{x}}-1)\]and \[\log ({{2}^{x}}+3)\]are in A.P., then \[x\] is equal to:
A)
5/2
done
clear
B)
log 25
done
clear
C)
log 23
done
clear
D)
logs 2
done
clear
View Answer play_arrow
question_answer 129) The set of all \[2\times 2\] matrices over the real numbers is not a group under matrix multiplication because:
A)
identity element does not exist
done
clear
B)
closure property is not satisfied
done
clear
C)
association property is not satisfied
done
clear
D)
inverse axiom may not be satisfied
done
clear
View Answer play_arrow
question_answer 130) The sum of the products of elements of any row of a determinant A with the same row is always equal to:
A)
1
done
clear
B)
zero
done
clear
C)
\[|A|\]
done
clear
D)
\[|A|/2\]
done
clear
View Answer play_arrow
question_answer 131) The direction cosines of the vector\[3\vec{i}-4\vec{j}+5\vec{k}\]are:
A)
\[\frac{3}{5},\frac{-4}{5},\frac{1}{5}\]
done
clear
B)
\[\frac{3}{5\sqrt{2}},\frac{-4}{5\sqrt{2}},\frac{1}{\sqrt{2}}\]
done
clear
C)
\[\frac{3}{\sqrt{2}},\frac{-4}{\sqrt{2}},\frac{1}{\sqrt{2}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 132) If A, B, C are the vertices of a triangle whose position vectors are \[\vec{a},\vec{b},\vec{c}\]a and G the centroid of the \[\Delta A B C,\] then\[G\vec{A}+G\vec{B}+G\vec{C}=\]
A)
\[\vec{0}\]
done
clear
B)
\[\vec{A}+\vec{B}+\vec{C}\]
done
clear
C)
\[\frac{\vec{a}+\vec{b}+\vec{c}}{3}\]
done
clear
D)
\[\frac{\vec{a}-\vec{b}-\vec{c}}{3}\]
done
clear
View Answer play_arrow
question_answer 133) If \[\underset{x\to \infty }{\mathop{\lim }}\,\left[ \frac{{{x}^{3}}+1}{{{x}^{2}}+1}-(ax-b) \right]=2,\]then:
A)
\[a=1\]and \[b=1\]
done
clear
B)
\[a=1\]and \[b=-1\]
done
clear
C)
\[a=1\]and \[b=-2\]
done
clear
D)
\[a=1\]and \[b=2\]
done
clear
View Answer play_arrow
question_answer 134) \[\underset{x\to 1}{\mathop{\lim }}\,\frac{1+\log x-x}{1-2x+{{x}^{2}}}=\]
A)
1
done
clear
B)
\[-1\]
done
clear
C)
zero
done
clear
D)
\[-1/2\]
done
clear
View Answer play_arrow
question_answer 135) The sum of all positive divisors of 960 is:
A)
3048
done
clear
B)
3087
done
clear
C)
3047
done
clear
D)
2180
done
clear
View Answer play_arrow
question_answer 136) If \[\vec{a},\vec{b},\vec{c}\]are unit vectors, such that\[\vec{a}+\vec{b}+\vec{c}=0\] then value of \[\vec{a}.\vec{b}+\vec{b}.\vec{c}+\vec{c}.\vec{a}\]is:
A)
\[3/2\]
done
clear
B)
\[-3/2\]
done
clear
C)
\[2/3\]
done
clear
D)
\[-2/3\]
done
clear
View Answer play_arrow
question_answer 137) If the vectors \[\vec{i}+3\vec{j}-2\vec{k},2\vec{i}-\vec{j}+4\vec{k}\]and \[3\vec{i}+2\vec{j}+x\vec{k}\]are coplanar, then the value of \[x\] is:
A)
\[-2\]
done
clear
B)
2
done
clear
C)
1
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 138) The radius of the circle \[{{x}^{2}}+{{y}^{2}}+4x-6y-12=0\]is:
A)
9
done
clear
B)
5
done
clear
C)
\[-3\]
done
clear
D)
\[-6\]
done
clear
View Answer play_arrow
question_answer 139) The equation of a circle parsing through (1, 0) and (0, 1) and having smallest possible radius is:
A)
\[{{x}^{2}}+{{y}^{2}}-x-y=0\]
done
clear
B)
\[{{x}^{2}}+{{y}^{2}}+x+y=0\]
done
clear
C)
\[2{{x}^{2}}+{{y}^{2}}-2x-y=0\]
done
clear
D)
\[{{x}^{2}}+2{{y}^{2}}-x-2y=0\]
done
clear
View Answer play_arrow
question_answer 140) If g is the inverse function of \[f\] and \[f'(x)=\frac{1}{1+{{x}^{n}}},\] then the value of\[g'(x)=\]
A)
\[{{(g(x))}^{n}}\]
done
clear
B)
\[1+{{(g(x))}^{n}}\]
done
clear
C)
\[1-{{(g(x))}^{n}}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 141) If\[\cos A=\cos B=\cos C\]and \[A+B+C=\pi ,\]then \[\cot B\cot C\]will be equal to:
A)
1/2
done
clear
B)
5/4
done
clear
C)
\[SinA\sin B\]
done
clear
D)
\[\cos A\cos B\]
done
clear
View Answer play_arrow
question_answer 142) If \[{{P}_{n}}={{\cos }^{n}}\theta +{{\sin }^{n}}\theta ,\]then
A)
1
done
clear
B)
zero
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 143) The radical axis of the circles belong to the coaxial system of circles, whose limiting points are (1, 3) and (2, 6) is:
A)
\[x-3y-15=0\]
done
clear
B)
\[x+3y-15=0\]
done
clear
C)
\[x-3y+15=0\]
done
clear
D)
\[2x+3y-16=0\]
done
clear
View Answer play_arrow
question_answer 144) If the latus ractum of an ellipse is one half of its minor axis then its eccentricity is equal to:
A)
\[\sqrt{3}/2\]
done
clear
B)
\[\sqrt{3}/4\]
done
clear
C)
1/2
done
clear
D)
\[1/\sqrt{2}\]
done
clear
View Answer play_arrow
question_answer 145) A force \[\vec{F}=2\vec{i}+\vec{j}-\vec{k}\]acts at a point A whose position vector is \[2\vec{i}-\vec{j}.\] The moment of \[\vec{F}\]about the origin is:
A)
\[\vec{i}+2\vec{j}-4\vec{k}\]
done
clear
B)
\[\vec{i}-2\vec{j}-4\vec{k}\]
done
clear
C)
\[\vec{i}+2\vec{j}+4\vec{k}\]
done
clear
D)
\[\vec{i}-2\vec{j}+4\vec{k}\]
done
clear
View Answer play_arrow
question_answer 146) Identify the false statement:
A)
\[(G,*),ab=ac\Rightarrow b=c\forall a,b,c\in C\]
done
clear
B)
In an abelian group,\[{{(ab)}^{2}}={{a}^{2}}{{b}^{2}},\forall a,b,\in G\]
done
clear
C)
Cube roots of unity forms an additive abelian group
done
clear
D)
In a group of even order, there exist an element other than identity which is its own inverse.
done
clear
View Answer play_arrow
question_answer 147) \[\underset{x\to 0}{\mathop{\lim }}\,\frac{{{a}^{\sin x}}-1}{{{b}^{\sin x}}-1}\]is equal to:
A)
a/b
done
clear
B)
b/a
done
clear
C)
\[\frac{\log a}{\log b}\]
done
clear
D)
\[\frac{\log b}{\log a}\]
done
clear
View Answer play_arrow
question_answer 148) The coefficient of the term independent of \[x\] in the expansion of\[{{\left( \sqrt{x/3}+\frac{3}{2{{x}^{2}}} \right)}^{10}}\]is: \[{{\left( \sqrt{x/3}+\frac{3}{2{{x}^{2}}} \right)}^{10}}\]is:
A)
\[5/4\]
done
clear
B)
7/4
done
clear
C)
\[9/4\]
done
clear
D)
\[3/4\]
done
clear
View Answer play_arrow
question_answer 149) If \[2\sec 2\alpha =\tan \beta +\cot \beta ,\] then one the value of \[(\alpha +\beta )\] is:
A)
\[\pi /4\]
done
clear
B)
\[\pi /2\]
done
clear
C)
\[\pi \]
done
clear
D)
\[2\pi \]
done
clear
View Answer play_arrow
question_answer 150) If \[{{(x+iy)}^{1/3}}=a+ib,\]then \[\frac{x}{a}+\frac{y}{b}=\]
A)
\[ab\]
done
clear
B)
\[4ab\]
done
clear
C)
\[4({{a}^{2}}-{{b}^{2}})\]
done
clear
D)
\[4({{a}^{2}}+{{b}^{2}})\]
done
clear
View Answer play_arrow
question_answer 151) The derivative of \[{{\sin }^{-1}}\left( \frac{2x}{1+{{x}^{2}}} \right)\] w.r.t.\[{{\cos }^{-1}}\left( \frac{1-{{x}^{2}}}{1+{{x}^{2}}} \right)\]is:
A)
\[-1\]
done
clear
B)
\[1\]
done
clear
C)
2
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 152) If R is the radius of circum circle of the\[\Delta ABC\] and \[\Delta \]is its area, then:
A)
\[R=\frac{a+b+c}{\Delta }\]
done
clear
B)
\[R=\frac{a+b+c}{4\Delta }\]
done
clear
C)
\[R=\frac{abc}{4\Delta }\]
done
clear
D)
\[R=\frac{abc}{\Delta }\]
done
clear
View Answer play_arrow
question_answer 153) \[\int_{{}}^{{}}{\frac{(x+3){{e}^{x}}}{{{(x+4)}^{2}}}}dx\]
A)
\[\frac{1}{{{(x+4)}^{2}}}+c\]
done
clear
B)
\[\frac{{{e}^{x}}}{{{(x+4)}^{2}}}+c\]
done
clear
C)
\[\frac{{{e}^{x}}}{x+4}+c\]
done
clear
D)
\[\frac{{{e}^{x}}}{x+3}+c\]
done
clear
View Answer play_arrow
question_answer 154) \[\int_{0}^{2a}{\frac{f(x)}{f(x)+f(2a-x)}dx}\]
A)
\[\pi /4\]
done
clear
B)
\[\pi /2\]
done
clear
C)
\[a\]
done
clear
D)
\[2a\]
done
clear
View Answer play_arrow
question_answer 155) The differential equation obtained on eliminating A and B from the equation \[y=A\cos \omega t+B\sin \omega t\]
A)
\[y''=-{{\omega }^{2}}y\]
done
clear
B)
\[y''+y=0\]
done
clear
C)
\[y''+y'=0\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 156) The solution of \[\frac{dy}{dx}={{2}^{y-x}}\]is:
A)
\[{{2}^{x}}+{{2}^{y}}=c\]
done
clear
B)
\[{{2}^{x}}-{{2}^{y}}=c\]
done
clear
C)
\[\frac{1}{{{2}^{x}}}-\frac{1}{{{2}^{y}}}=c\]
done
clear
D)
\[\frac{1}{{{2}^{x}}}+\frac{1}{{{2}^{y}}}=c\]
done
clear
View Answer play_arrow
question_answer 157) \[\int_{{}}^{{}}{\frac{\sin xdx}{3+4{{\cos }^{2}}x}}\]
A)
\[\log (3+4{{\cos }^{2}}x)+c\]
done
clear
B)
\[-\frac{1}{2\sqrt{3}}{{\tan }^{-1}}\left( \frac{\cos x}{\sqrt{3}} \right)+c\]
done
clear
C)
\[-\frac{1}{2\sqrt{3}}{{\tan }^{-1}}\left( \frac{2\cos x}{\sqrt{3}} \right)+c\]
done
clear
D)
\[\frac{1}{2\sqrt{3}}{{\tan }^{-1}}\left( \frac{2\cos x}{\sqrt{3}} \right)+c\]
done
clear
View Answer play_arrow
question_answer 158) The value of\[\int_{-1}^{3}{\left[ {{\tan }^{-1}}\left( \frac{x}{{{x}^{2}}+1} \right)+{{\tan }^{-1}}\left( \frac{{{x}^{2}}+1}{x} \right) \right]dx}\]is
A)
\[2\pi \]
done
clear
B)
\[\pi \]
done
clear
C)
\[\pi /2\]
done
clear
D)
\[\pi /4\]
done
clear
View Answer play_arrow
question_answer 159) \[\int_{0}^{\pi /2}{\log (\tan x)dx}\]
A)
zero
done
clear
B)
2
done
clear
C)
\[\pi /3\]
done
clear
D)
\[\pi /4\]
done
clear
View Answer play_arrow
question_answer 160) \[\int_{0}^{\pi /4}{\log (1+\tan x)\,dx}\]
A)
\[\frac{\pi }{4}\log 2\]
done
clear
B)
\[\frac{\pi }{8}\log 2\]
done
clear
C)
\[\frac{\pi }{4}\log \tan x\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 161) The maximum value of \[\frac{{{\log }_{e}}x}{x}\]in\[0<x<\infty \]is:
A)
\[e\]
done
clear
B)
\[{{\log }_{x}}e\]
done
clear
C)
\[1/e\]
done
clear
D)
\[e/2\]
done
clear
View Answer play_arrow
question_answer 162) If \[{{x}_{r}}=\cos \frac{\pi }{{{2}^{r}}}+i\sin \frac{\pi }{{{2}^{r}}},\]then\[{{x}_{1}},{{x}_{2}},{{x}_{3...\infty }}\]is equal to:
A)
zero
done
clear
B)
1
done
clear
C)
\[\pi \]
done
clear
D)
\[-1\]
done
clear
View Answer play_arrow
question_answer 163) Which of the following is the empty set:
A)
[\[x:x\]is a real number and\[{{x}^{2}}-1=0\,\]}
done
clear
B)
[\[x:x\]is a real number and \[{{x}^{2}}+1=0\]}
done
clear
C)
[\[x:x\]is a real number and\[{{x}^{2}}-9=0\]}
done
clear
D)
[\[x:x\]is a real number and\[{{x}^{2}}=x+2\]}
done
clear
View Answer play_arrow
question_answer 164) If \[A=\{1,2\}\]and \[B=(0,1)\]then \[A\times B\]is:
A)
\[\{(1,0),(1,1),(2,0),(2,1)\}\]
done
clear
B)
\[\{(1,0),(2,1)\}\]
done
clear
C)
\[\{(1,1),(1,2),(0,1),(0,2)\}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 165) \[f:R\to R\]is a function defined by\[f(x)=10x-7.\]If \[g={{f}^{-1}}\]then \[g(x)=\]
A)
\[\frac{1}{10{{x}^{-7}}}\]
done
clear
B)
\[\frac{1}{10x+7}\]
done
clear
C)
\[\frac{x+7}{10}\]
done
clear
D)
\[\frac{x-7}{10}\]
done
clear
View Answer play_arrow
question_answer 166) The identity element for the binary operation * defined by \[a*b=\frac{ab}{2},a,b\in {{Q}_{0}}\] (the set of all non-zero rational number) is:
A)
1
done
clear
B)
zero
done
clear
C)
2
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 167) The curve represented by\[\operatorname{Im}({{z}^{2}})=K,\]where K is a non-zero real number is:
A)
a pair of straight lines
done
clear
B)
an ellipse
done
clear
C)
a parabola
done
clear
D)
a hyperbola
done
clear
View Answer play_arrow
question_answer 168) The value of \[{{i}^{i}}\]is:
A)
\[\omega \]
done
clear
B)
\[-{{\omega }^{2}}\]
done
clear
C)
\[\pi /2\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 169) If 7th and 13th terms of an AP be 34 and 64 respectively, then its 18th term is:
A)
87
done
clear
B)
88
done
clear
C)
89
done
clear
D)
90
done
clear
View Answer play_arrow
question_answer 170) If \[\alpha ,\beta \]are the roots of the equation x2 + x +1 \[{{x}^{2}}+x+1=0\]and \[\alpha /\beta ,\beta /\alpha \]are roots of the equation \[{{x}^{2}}+px+q=0\]then p equals:
A)
\[-1\]
done
clear
B)
\[1\]
done
clear
C)
\[-2\]
done
clear
D)
\[2\]
done
clear
View Answer play_arrow
question_answer 171) The number of ways to arrange the letters of word CHEESE are:
A)
120
done
clear
B)
240
done
clear
C)
720
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 172) The coefficient of\[{{x}^{4}}\]in the expansion of \[{{(1+x+{{x}^{2}}+{{x}^{3}})}^{11}}\]is:
A)
900
done
clear
B)
909
done
clear
C)
990
done
clear
D)
999
done
clear
View Answer play_arrow
question_answer 173) If A and B are two matrices such that \[AB=B\]and \[BA=A\]then \[{{A}^{2}}+{{B}^{2}}=\]
A)
\[2AB\]
done
clear
B)
\[2BA\]
done
clear
C)
\[A\div B\]
done
clear
D)
\[AB\]
done
clear
View Answer play_arrow
question_answer 174) The value of the determinant\[\left| \begin{matrix} 1 & {{\omega }^{2}} & {{\omega }^{5}} \\ {{\omega }^{2}} & 1 & {{\omega }^{4}} \\ {{\omega }^{5}} & {{\omega }^{4}} & 1 \\ \end{matrix} \right|\]where \[\omega \]is an imaginary cube root of unity is:
A)
\[{{(1-\omega )}^{2}}\]
done
clear
B)
3
done
clear
C)
\[-3\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 175) The line \[x+y=4\]divides the line joining\[(-1,1)\] and \[(5,7)\] in the ratio\[\lambda :1,\] then the value of\[\lambda \] is:
A)
2
done
clear
B)
\[1/2\]
done
clear
C)
3
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 176) Four distinct points \[(2K,3K),(1,0),(0,1)\] and (0, 0) lie on a circle for:
A)
all integral value of K
done
clear
B)
\[0<K<1\]
done
clear
C)
\[K<0\]
done
clear
D)
for two values of K
done
clear
View Answer play_arrow
question_answer 177) The equation to the ellipse referred to its axes as the axes of\[x\]and \[y\]respectively) whose foci are \[(\pm \,2,0)\] and eccentricity 1/2 is:
A)
\[\frac{{{x}^{2}}}{12}+\frac{{{y}^{2}}}{16}=1\]
done
clear
B)
\[\frac{{{x}^{2}}}{16}+\frac{{{y}^{2}}}{12}=1\]
done
clear
C)
\[\frac{{{x}^{2}}}{16}+\frac{{{y}^{2}}}{8}=1\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 178) If \[f(x)=\frac{x-1}{x+1},\]then \[f(2x)\]is:
A)
\[\frac{f(x)+1}{f(x)+3}\]
done
clear
B)
\[\frac{3f(x)+1}{f(x)+3}\]
done
clear
C)
\[\frac{f(x)+3}{f(x)+1}\]
done
clear
D)
\[\frac{f(x+3)}{3f(x)+1}\]
done
clear
View Answer play_arrow
question_answer 179) The degree of the differential equation \[{{\left( \frac{{{d}^{2}}y}{d{{x}^{2}}} \right)}^{2}}+{{\left( \frac{dy}{dx} \right)}^{2}}=x\sin \left( \frac{{{d}^{2}}y}{dx} \right)\]is:
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 180) In any group the number of improper subgroups is:
A)
2
done
clear
B)
3
done
clear
C)
Depends on the given group
done
clear
D)
1
done
clear
View Answer play_arrow
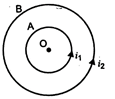 currents\[{{i}_{1}}\]and\[{{i}_{2}}\]as shown in the diagram. If ratio of their radii is 1 : 2 and ratio of the flux densities at O due to A and is 1 : 3 then the value of \[\frac{{{i}_{1}}}{{{i}_{2}}}\] will be:
currents\[{{i}_{1}}\]and\[{{i}_{2}}\]as shown in the diagram. If ratio of their radii is 1 : 2 and ratio of the flux densities at O due to A and is 1 : 3 then the value of \[\frac{{{i}_{1}}}{{{i}_{2}}}\] will be:
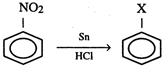 In the above reaction X stands for:
In the above reaction X stands for:
