question_answer 1) Which of the following pairs have identical dimensions?
A)
Momentum and force
done
clear
B)
Pressure and surface tension
done
clear
C)
Moment of force and angular momentum
done
clear
D)
Surface tension and surface energy
done
clear
View Answer play_arrow
question_answer 2) A train 100 m long travelling at 40 m/s starts overtaking another train 200 m long travelling at 30 m/s. The time taken by the first train to pass the second train completely is
A)
30s
done
clear
B)
40s
done
clear
C)
50s
done
clear
D)
60s
done
clear
View Answer play_arrow
question_answer 3) A constant power P is applied to a particle of mass m. The distance travelled by the particle when its velocity increases from \[{{v}_{1}}\] to \[{{v}_{2}}\] is (neglect friction)
A)
\[\frac{m}{3P}(v_{2}^{3}-v_{1}^{3})\]
done
clear
B)
\[\frac{m}{3P}({{v}_{2}}-{{v}_{1}})\]
done
clear
C)
\[4.32\times {{10}^{6}}\]
done
clear
D)
\[\frac{m}{3P}(v_{2}^{2}-v_{1}^{2})\]
done
clear
View Answer play_arrow
question_answer 4) A spring of constant \[5\times {{10}^{3}}\] N/m is stretched initially by 5 cm from the unstretched position. Then the work required to stretch it further by another 5 cm is
A)
6.25 Nm
done
clear
B)
12.5 Nm
done
clear
C)
18.75 Nm
done
clear
D)
25.00 Nm
done
clear
View Answer play_arrow
question_answer 5) Four spheres each having mass m and radius rare placed with their centres on the four corners of a square of side a. Then the moment of inertia of the system about an axis along one of the sides of the square is
A)
\[\frac{8}{5}\,m{{r}^{2}}\]
done
clear
B)
\[\frac{8}{5}\,m{{r}^{2}}+m{{a}^{2}}\]
done
clear
C)
\[\frac{8}{5}\,m{{r}^{2}}+2\,\,m{{a}^{2}}\]
done
clear
D)
\[\frac{4}{5}\,m{{r}^{2}}+4\,\,m{{a}^{2}}\]
done
clear
View Answer play_arrow
question_answer 6) If an artificial satellite is moving in a circular orbit around the earth with a speed equal to half the magnitude of the escape velocity from the earth, the height of the satellite above the surface of the earth is
A)
2R
done
clear
B)
R
done
clear
C)
R
done
clear
D)
R
done
clear
View Answer play_arrow
question_answer 7) A 2 kg copper block is heated to \[{{500}^{o}}C\] and then it is placed on a large block of ice at \[{{0}^{o}}C\]. If the specific heat capacity of copper is 400 J/kg/C and latent heat of water is \[3.5\times {{10}^{5}}\] J/kg\ The amount of ice that can melt is
A)
7/8 kg
done
clear
B)
7/5 kg
done
clear
C)
8/7 kg
done
clear
D)
5/7 kg
done
clear
View Answer play_arrow
question_answer 8) Number of waves in 8 cm of vacuum is same as number of waves in \[x\] cm of a medium. Refractive index of medium is 4/3, then the value \[x\] is
A)
32/3 cm
done
clear
B)
12cm
done
clear
C)
6 cm
done
clear
D)
4 cm
done
clear
View Answer play_arrow
question_answer 9) Consider a gas with density p and c as the root mean square velocity of its molecules contained in a volume. If the system moves as whole with velocity v, then the pressure exerted by the gas is
A)
\[\frac{1}{3}\rho {{\overline{c}}^{2}}\]
done
clear
B)
\[\frac{1}{3}\rho {{(c+v)}^{2}}\]
done
clear
C)
\[\frac{1}{3}\rho {{(\overline{c}-v)}^{2}}\]
done
clear
D)
\[\frac{1}{3}\rho \,\,{{(\overline{c}-v)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 10) At \[{{127}^{o}}C\] radiated energy is \[2.7\times {{10}^{-3}}\] J/s. At what temperature radiated energy is \[4.32\times {{10}^{6}}\]J/s?
A)
400 K
done
clear
B)
4000 K
done
clear
C)
80000 K
done
clear
D)
40000 K
done
clear
View Answer play_arrow
question_answer 11) The minimum phase difference between two simple harmonic oscillations, \[{{y}_{1}}=\frac{1}{2}\sin \omega \,t+\frac{\sqrt{3}}{2}\cos \,\omega \,t\] \[{{y}_{2}}=\sin \omega \,t+\cos \,\omega \,t\], is
A)
\[\frac{7\pi }{12}\]
done
clear
B)
\[\frac{\pi }{12}\]
done
clear
C)
\[\frac{-\pi }{6}\]
done
clear
D)
\[\frac{\pi }{6}\]
done
clear
View Answer play_arrow
question_answer 12) A rubber cord catapult has cross-sectional area 25 mm2 and initial length of rubber cord is 10 cm. It is stretched to 5 cm and then released to project a missile of mass 5 g. Taking\[{{Y}_{ruber}}=5\times {{10}^{8}}N{{m}^{-2}}\], velocity of projected missile is
A)
20 \[m{{s}^{-1}}\]
done
clear
B)
100 \[m{{s}^{-1}}\]
done
clear
C)
250 \[m{{s}^{-1}}\]
done
clear
D)
200 \[m{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 13) The material of a wire has a density of 1.4 g/\[c{{m}^{3}}\]. If it is not wetted by a liquid of surface tension 44 dyne/cm, then the maximum radius of the wire which can float on the surface of liquid is
A)
\[\frac{10}{28}\]cm
done
clear
B)
\[\frac{10}{14}\]cm
done
clear
C)
\[\frac{10}{7}\]mm
done
clear
D)
0.7 cm
done
clear
View Answer play_arrow
question_answer 14) Binding energy of satellite is \[4\times {{10}^{8}}J\], Its PE is
A)
\[-4\times {{10}^{8}}J\]
done
clear
B)
\[-8\times {{10}^{8}}J\]
done
clear
C)
\[8\times {{10}^{8}}J\]
done
clear
D)
\[4\times {{10}^{8}}J\]
done
clear
View Answer play_arrow
question_answer 15) A current of 0.01 mA passes through the potentiometer wire of a resistivity of \[{{10}^{9}}\Omega \] cm and area of cross-section \[{{10}^{-2}}\,c{{m}^{2}}\]. The potential gradient is
A)
\[{{10}^{9}}V/m\]
done
clear
B)
\[{{10}^{11}}V/m\]
done
clear
C)
\[{{10}^{10}}V/m\]
done
clear
D)
\[{{10}^{8}}V/m\]
done
clear
View Answer play_arrow
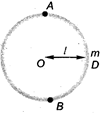
question_answer 16)
A particle of mass m attached with a string of length I is just revolving on the vertical circle without slacking of the string. If \[{{v}_{A}}\], Vg and \[{{v}_{D}}\]are speeds at positions A, B and D, then
A)
\[{{v}_{B}}>{{v}_{D}}>{{v}_{A}}\]
done
clear
B)
tension in string at D = 3 mg
done
clear
C)
\[{{v}_{D}}=\sqrt{3gl}\]
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 17) A bucket of water is being revolved in vertical circle of radius 1 m. Minimum frequency required to prevent the. water from getting down the path is \[(g=10\,\,m/{{s}^{2}})\]
A)
\[\frac{2\pi }{\sqrt{10}}\]
done
clear
B)
\[\frac{2\pi }{\sqrt{5}}\]
done
clear
C)
\[\frac{\sqrt{10}}{2\pi }\]
done
clear
D)
\[\frac{\sqrt{5}}{2\pi }\]
done
clear
View Answer play_arrow
question_answer 18) A round disc of moment of inertia \[{{I}_{2}}\] about its axis perpendicular to its plane and passing through its centre is placed over another disc of moment of inertia \[{{l}_{1}}\] rotating with an angular velocity co about the same axis. The final angular velocity of the combination of discs is
A)
\[\frac{{{I}_{2}}\,\,\omega }{{{I}_{1}}+{{I}_{2}}}\]
done
clear
B)
\[\omega \]
done
clear
C)
\[\frac{{{I}_{1}}\,\,\omega }{{{I}_{1}}+{{I}_{2}}}\]
done
clear
D)
\[\frac{({{I}_{1}}+{{I}_{2}})\,\omega }{{{I}_{1}}}\]
done
clear
View Answer play_arrow
question_answer 19) Two discs have same mass and thickness Their materials are of densities \[{{\rho }_{1}}\] and \[{{\rho }_{2}}\]. The ratio of their moment of inertia about central axis will be
A)
\[{{\rho }_{1}}:{{\rho }_{2}}\]
done
clear
B)
\[{{\rho }_{1}}:{{\rho }_{2}}:1\]
done
clear
C)
\[1:{{\rho }_{1}}\,\,{{\rho }_{2}}\]
done
clear
D)
\[{{\rho }_{2}}:{{\rho }_{1}}\]
done
clear
View Answer play_arrow
question_answer 20) A 4 m long wire of resistance \[8\,\Omega \] is connected in series with a battery of emf 2 V and a resistor of \[7\,\Omega \]. The internal resistance of the battery is \[1\,\,\Omega \]. What is the potential gradient along the wire?
A)
1, 00 \[V{{m}^{-1}}\]
done
clear
B)
0,75 \[V{{m}^{-1}}\]
done
clear
C)
0.50 \[V{{m}^{-1}}\]
done
clear
D)
0.25 \[V{{m}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 21) A uniform wire of \[16\,\,\Omega \] resistance is made into the form of a square. Two opposite corners of the square are connected by a wire of resistance \[16\,\,\Omega \]. The effective resistance between the other two opposite corners is
A)
\[32\,\,\Omega \]
done
clear
B)
\[16\,\,\Omega \]
done
clear
C)
\[8\,\,\Omega \]
done
clear
D)
\[4\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 22) A \[6\times {{10}^{-4}}F\] parallel plate air capacitor is connected to a 500 V battery. When air is replaced by another dielectric material, \[7.5\times {{10}^{-4}}C\] charge flows into the capacitor. The value of the dielectric constant of the material is
A)
1.5
done
clear
B)
2.0
done
clear
C)
1.0025
done
clear
D)
3.5
done
clear
View Answer play_arrow
question_answer 23) The 90 pF capacitor is connected to a 12 V battery. How many electrons are transferred from one plate to another?
A)
\[1.1\times {{10}^{9}}\]
done
clear
B)
\[6.7\times {{10}^{9}}\]
done
clear
C)
\[4\times {{10}^{19}}\]
done
clear
D)
\[5\times {{10}^{19}}\]
done
clear
View Answer play_arrow
question_answer 24) Given mass number of gold =197, Density of gold = 19.7 \[g/c{{m}^{3}}\] Avogadros number \[=6\times {{10}^{23}}\]. The radius of the gold atom is approximately
A)
\[1.5\times {{10}^{-8}}m\]
done
clear
B)
\[1.7\times {{10}^{-9}}m\]
done
clear
C)
\[1.5\times {{10}^{-10}}m\]
done
clear
D)
\[1.5\times {{10}^{-12}}m\]
done
clear
View Answer play_arrow
question_answer 25) In Youngs double slit experiment, two slits are separated by 1 m. The slits are illuminated by a light of wavelength 650 nm. The source of light is placed symmetrically with respect to the two slits. Interference pattern is observed on a screen at a distance of 1 m from the slits. The distance between the third dark fringe and the fifth bright fringe from the centre of the pattern will be
A)
1.62 mm
done
clear
B)
2.62 mm
done
clear
C)
5.62 mm
done
clear
D)
3.62 mm
done
clear
View Answer play_arrow
question_answer 26) R is a radius of a planet and p is its density. The escape velocity on its surface will be
A)
\[{{R}^{2}}\sqrt{4\pi G\rho /3}\]
done
clear
B)
\[R\sqrt{4\pi G\rho /3}\]
done
clear
C)
\[{{R}^{2}}\sqrt{8\pi G\rho /3}\]
done
clear
D)
\[R\sqrt{8\pi G\rho /3}\]
done
clear
View Answer play_arrow
question_answer 27) A satellite is moving in a circular orbit at a certain height above the earths surface. It takes \[5.26\times {{10}^{3}}s\] to complete one revolution with a centripetal acceleration equal to 9.32\[m/{{s}^{2}}\]. The height of satellite orbiting above the earth is (Earths radius \[=6.37\times {{10}^{6}}m\])
A)
220 km
done
clear
B)
160 km
done
clear
C)
70km
done
clear
D)
120 km
done
clear
View Answer play_arrow
question_answer 28) The surface tension of soap solution is 0.03 N/m. The amount of work done in forming a bubble of radius 5 cm is
A)
3.77 J
done
clear
B)
1.885 J
done
clear
C)
\[0.95\times {{10}^{-3}}J\]
done
clear
D)
\[1.9\times {{10}^{-3}}J\]
done
clear
View Answer play_arrow
question_answer 29) The minimum velocity of capillary waves on the surface of water is (surface tension of water \[=7.2\times {{10}^{-2}}\] N/m)
A)
0.23 m/s
done
clear
B)
0.46 m/s
done
clear
C)
0.69 m/s
done
clear
D)
0.92 m/s
done
clear
View Answer play_arrow
question_answer 30) A wire has a breaking stress of \[6\times {{10}^{5}}N/{{m}^{2}}\] and a density of \[3\times {{10}^{4}}kg/{{m}^{3}}\]. The length of the wire of the same material which will break under its own weight, (if \[g=10\,m/{{s}^{2}}\]) is
A)
2000m
done
clear
B)
2500m
done
clear
C)
20m
done
clear
D)
2m
done
clear
View Answer play_arrow
question_answer 31) A man measures the period of simple pendulum inside a stationary-lift and finds it to be T second. If the lift accelerates downwards with acceleration of \[\frac{g}{4}\], the period of oscillation will be
A)
\[T\times \frac{\sqrt{3}}{2}\,s\]
done
clear
B)
\[T\times \frac{2}{\sqrt{3}}\,s\]
done
clear
C)
\[\frac{T}{2}s\]
done
clear
D)
\[\sqrt{T}\,s\]
done
clear
View Answer play_arrow
question_answer 32) A spherical black body with a radius of 12 cm radiates 450 W power at 500 K. If the radius were halved and temperature be doubled, the power radiated in watt would be
A)
1800
done
clear
B)
900
done
clear
C)
3600
done
clear
D)
850
done
clear
View Answer play_arrow
question_answer 33) If the emissive power of black surface at same temperature is 400 \[W/{{m}^{2}}\], the emissive and absorptive powers of the surface assuming it was initially ordinary surface, are (Given, Mass of the body m = 4.2 kg, Area of body \[=5\times {{10}^{-2}}{{m}^{2}}\], Rate of cooling \[\frac{d\theta }{dt}=\frac{1}{12}\times {{10}^{-2}}{{\,}^{o}}C/\min \] Specific heat \[s=420J/kg{{\,}^{o}}C\])
A)
e = a = 0.0735
done
clear
B)
e = a = 0.0435
done
clear
C)
e = a = 0.0535
done
clear
D)
e = a = 0.0235
done
clear
View Answer play_arrow
question_answer 34) The expression for total kinetic energy per unit volume of gas is
A)
\[\frac{E}{V}=\frac{p}{2}\]
done
clear
B)
\[\frac{E}{V}=\frac{1}{3}p\]
done
clear
C)
\[\frac{E}{V}=\frac{2}{3}p\]
done
clear
D)
\[\frac{E}{V}=\frac{3}{2}p\]
done
clear
View Answer play_arrow
question_answer 35) A vessel contains a mixture of one mole of oxygen and two moles of nitrogen at 300 K. The ratio of the average rotational KE per oxygen molecule that per nitrogen molecule is
A)
1 : 1
done
clear
B)
1 : 2
done
clear
C)
2 : 1
done
clear
D)
depends on the moments of inertia of the two molecules
done
clear
View Answer play_arrow
question_answer 36) The wavelength and frequency of beam of light in water of refractive index 4/3 having wavelength 0.48 micron in air are
A)
\[0.16\times {{10}^{-6}}m,\,\,6.25\times {{10}^{14}}Hz\]
done
clear
B)
\[0.36\times {{10}^{-6}}m,\,\,6.25\times {{10}^{14}}Hz\]
done
clear
C)
\[0.36\times {{10}^{-6}}m,\,\,3.25\times {{10}^{14}}Hz\]
done
clear
D)
\[0.26\times {{10}^{-6}}m,\,\,6.25\times {{10}^{14}}Hz\]
done
clear
View Answer play_arrow
question_answer 37) A ray of light is incident on glass slab making an angle of incidence \[{{\sin }^{-1}}\left( \frac{\sqrt{3}}{2} \right)\]. What will be the angle of refraction in glass of refractive index 1.5?
A)
\[{{40}^{o}}\,18\]
done
clear
B)
\[{{24}^{o}}\,49\]
done
clear
C)
\[{{25}^{o}}\,17\]
done
clear
D)
\[{{35}^{o}}\,16\]
done
clear
View Answer play_arrow
question_answer 38) An electron at rest is accelerated through a potential difference of 200 V. If the electron acquires a velocity \[8.4\times {{10}^{6}}\] m/s, the value of e/m of electron is
A)
\[1.76\times {{10}^{-4}}C/kg\]
done
clear
B)
\[1.76\times {{10}^{14}}C/kg\]
done
clear
C)
\[1.76\times {{10}^{11}}C/kg\]
done
clear
D)
\[1.76\times {{10}^{-16}}C/kg\]
done
clear
View Answer play_arrow
question_answer 39) A radio transmitter operates at a frequency of 880 kHz and power of 10 kW. The number of photons emitted per second is
A)
\[13.27\times {{10}^{4}}\]
done
clear
B)
\[13.27\times {{10}^{34}}\]
done
clear
C)
\[1327\times {{10}^{34}}\]
done
clear
D)
\[1.71\times {{10}^{31}}\]
done
clear
View Answer play_arrow
question_answer 40) What is the voltage gain in a common emitter amplifier, when input resistance is \[3\,\,\Omega \]. and load resistance is \[24\,\,\Omega \]. with \[\beta =60\]?
A)
480
done
clear
B)
2.4
done
clear
C)
4.8
done
clear
D)
8.4
done
clear
View Answer play_arrow
question_answer 41) The input resistance of a common emitter transistor amplifier, if the output resistance is\[500\,\,k\Omega \], the current gain \[\alpha =0.98\] and the power gain is \[6.0625\times {{10}^{6}}\], is
A)
\[198\,\Omega \]
done
clear
B)
\[300\,\Omega \]
done
clear
C)
\[100\,\Omega \]
done
clear
D)
\[400\,\Omega \]
done
clear
View Answer play_arrow
question_answer 42) Which of the following transitions in hydrogen atom produces longest wavelength of radiation (or photon of minimum energy)?
A)
n = 2, p = 4
done
clear
B)
n = 3, p = 4
done
clear
C)
n = 6, p = 8
done
clear
D)
n = 5, p = 6
done
clear
View Answer play_arrow
question_answer 43) If \[\left( \frac{0.51\times {{10}^{-10}}}{4} \right)\]m, is the radius of smallest electron orbit in hydrogen like atom, then this atom is
A)
H-atom
done
clear
B)
\[H{{e}^{+}}\]
done
clear
C)
\[l{{i}^{2+}}\]
done
clear
D)
\[B{{e}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 44) A series resonant circuit contains \[L=\frac{5}{\pi }\,mH\],\[C=\frac{200}{\pi }\mu F\] and \[R=100\,\,\Omega \]. If a source of emf \[e=200\sin 100\,\,\pi t\] is applied, then the rms current is
A)
2 A
done
clear
B)
\[200\sqrt{2}\,A\]
done
clear
C)
\[100\sqrt{2}\,A\]
done
clear
D)
1.41 A
done
clear
View Answer play_arrow
question_answer 45) A rod of length 1.0 m is rotated in a plane perpendicular to a uniform magnetic field of induction 0.25 T with a frequency of 12 rev/s. The induced emf across the ends of the rod is
A)
18.89V
done
clear
B)
3V
done
clear
C)
15V
done
clear
D)
9.42V
done
clear
View Answer play_arrow
question_answer 46) A ferromagnetic material is heated above its curie temperature. Which one is a correct statement?
A)
Ferromagnetic domains are perfectly arranged
done
clear
B)
Ferromagnetic domains become random
done
clear
C)
Ferromagnetic domains are not influenced
done
clear
D)
Ferromagnetic material changes into diamagnetic material
done
clear
View Answer play_arrow
question_answer 47) A material is placed in a magnetic field and it is thrown out of it. Then the material is
A)
paramagnetic
done
clear
B)
diamagnetic
done
clear
C)
ferromagnetic
done
clear
D)
non-magnetic
done
clear
View Answer play_arrow
question_answer 48) A charged particle of mass m and charge q is accelerated through a potential difference of V volt. It enters a region of uniform magnetic field which is directed perpendicular to the direction of mouon of the panicle. The particle will move on a circular path of radius given by
A)
\[\sqrt{\frac{Vm}{q{{B}^{2}}}}\]
done
clear
B)
\[\frac{Vm}{q{{B}^{2}}}\]
done
clear
C)
\[\sqrt{\frac{2Vm}{q}}\,\,.\,\left( \frac{1}{B} \right)\]
done
clear
D)
\[\sqrt{\frac{Vm}{q}}\,\,.\,\left( \frac{1}{B} \right)\]
done
clear
View Answer play_arrow
question_answer 49) An electron moves at right angle to a magnetic field of \[1.5\times {{10}^{-2}}T\] with a speed of \[6\times {{10}^{-7}}\]m/s. If the specific charge on the electron is \[1.7\times {{10}^{11}}\] C/kg, the radius of the circular path will be
A)
2.9 cm
done
clear
B)
3.9 cm
done
clear
C)
2.35cm
done
clear
D)
2cm
done
clear
View Answer play_arrow
question_answer 50) In a circuit, 5 percent of total current passes through a galvanometer. If resistance of the galvanometer is G. Then the value of the shunt is
A)
19 G
done
clear
B)
20 G
done
clear
C)
\[\frac{G}{20}\]
done
clear
D)
\[\frac{G}{19}\]
done
clear
View Answer play_arrow
question_answer 51) Given, C (diamond) \[+{{O}_{2}}\xrightarrow{{}}C{{O}_{2}};\Delta H=-395\,kJ\] C (graphite) \[+{{O}_{2}}\xrightarrow{{}}C{{O}_{2}};\Delta H=-393\,kJ\] The heat of formation of diamond from graphite is
A)
+2.0 kJ
done
clear
B)
- 1.5 kJ
done
clear
C)
-788 kJ
done
clear
D)
788 kJ
done
clear
View Answer play_arrow
question_answer 52) The type of isomerism observed in benzaldoxime is
A)
optical
done
clear
B)
functional
done
clear
C)
geometrial
done
clear
D)
tautomerism
done
clear
View Answer play_arrow
question_answer 53) Sodium metal is prepared commercially by electrolysis of fused \[NaCl\] by
A)
Downs process
done
clear
B)
Nelson cell
done
clear
C)
Solvay process
done
clear
D)
Castner and Kellners cell
done
clear
View Answer play_arrow
question_answer 54) The value of \[x\] is maximum for
A)
\[MgS{{O}_{4}}\,.\,\,x\,{{H}_{2}}O\]
done
clear
B)
\[CaS{{O}_{4}}\,.\,\,x\,{{H}_{2}}O\]
done
clear
C)
\[BaS{{O}_{4}}\,.\,\,x\,{{H}_{2}}O\]
done
clear
D)
All have the same value of \[x\]
done
clear
View Answer play_arrow
question_answer 55) Heating of ore-in absence of air below its melting point is called
A)
leaching
done
clear
B)
roasting
done
clear
C)
smelting
done
clear
D)
calcination
done
clear
View Answer play_arrow
question_answer 56) In fluorine group of Periodic Table, on going down
A)
ionic radius increases
done
clear
B)
electronegativity increases
done
clear
C)
ionisation potential increases
done
clear
D)
reactivity increases
done
clear
View Answer play_arrow
question_answer 57) The ether that undergoes electrophilic substitution reactions is
A)
\[C{{H}_{3}}O{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}OC{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}OC{{H}_{3}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 58) The order of reactivity of alcohols towards sodium metal is
A)
Primary > Secondary > Tertiary
done
clear
B)
Primary < Secondary < Tertiary
done
clear
C)
Primary < Secondary > Tertiary
done
clear
D)
Primary > Secondary < Tertiary
done
clear
View Answer play_arrow
question_answer 59) If one mole of a substance is present in 1 kg of solvent then its concentration is called
A)
molar cone
done
clear
B)
molal cone
done
clear
C)
normality
done
clear
D)
Strength wt/wt.
done
clear
View Answer play_arrow
question_answer 60) If the pressure and absolute temperature of 2L of carbondioxide gas are doubled, the value of the gas would become
A)
2L
done
clear
B)
4L
done
clear
C)
5 L
done
clear
D)
7L
done
clear
View Answer play_arrow
question_answer 61) \[C{{H}_{3}}CO{{O}^{-}}\] ion is a
A)
weak conjugate base
done
clear
B)
strong conjugate base
done
clear
C)
weak conjugate acid
done
clear
D)
strong conjugate acid
done
clear
View Answer play_arrow
question_answer 62) The hybrid state of C in charcoal is
A)
\[s{{p}^{3}}\]
done
clear
B)
\[s{{p}^{2}}\]
done
clear
C)
sp
done
clear
D)
No specific state
done
clear
View Answer play_arrow
question_answer 63) The shape of \[Cl{{F}_{3}}\] molecule is
A)
triangular planar
done
clear
B)
pyramidal
done
clear
C)
T-shape
done
clear
D)
trigonal bipyramidal
done
clear
View Answer play_arrow
question_answer 64) Equivalent weight of crystalline oxalic acid is
A)
90
done
clear
B)
63
done
clear
C)
53
done
clear
D)
45
done
clear
View Answer play_arrow
question_answer 65) If \[x\,mol\,\,{{L}^{-1}}\] is the solubility of \[KAl{{(S{{O}_{4}})}_{2}}\] then \[{{K}_{sp}}\] is equal to
A)
\[{{x}^{3}}\]
done
clear
B)
\[4{{x}^{4}}\]
done
clear
C)
\[{{x}^{4}}\]
done
clear
D)
\[4{{x}^{3}}\]
done
clear
View Answer play_arrow
question_answer 66) In the reaction, \[{{N}_{2}}(g)+{{O}_{2}}(g)2NO(g)-180.7\,kJ\] on increasing the temperature, the production of NO
A)
increases
done
clear
B)
decreases
done
clear
C)
remains same
done
clear
D)
Cannot be predicted
done
clear
View Answer play_arrow
question_answer 67) In an experiment, 20 mL of decinormal \[HCl\]solution was added to 10 mL of a decinormal \[AgN{{O}_{3}}\] solution. \[AgCl\] was precipitated out and the excess acid was titrated against a, decinormal \[NaOH\] solution. Volume of \[NaOH\]required for this back titration is
A)
10 mL
done
clear
B)
5 mL
done
clear
C)
20 mL
done
clear
D)
30 mL
done
clear
View Answer play_arrow
question_answer 68)
Consider the following statements. I. The colour of the hydrophobic sol depends on the wavelength of the light scattered by the dispersed particle. II. The smaller the gold number value of a hydrophilic colloid, the greater is its protective power. III. The movement of sol particle under an applied electric potential is called electroosmosis.
Which of the above statements are correct?
A)
I and II
done
clear
B)
I and III
done
clear
C)
II and III
done
clear
D)
I, II and III
done
clear
View Answer play_arrow
question_answer 69) Natural gas is
A)
\[CO+{{H}_{2}}\]
done
clear
B)
\[CO+{{N}_{2}}\]
done
clear
C)
\[C{{H}_{4}}+{{O}_{2}}+{{N}_{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 70) Colemanite is
A)
\[N{{a}_{2}}{{B}_{4}}{{O}_{7}}\,.\,10{{H}_{2}}O\]
done
clear
B)
\[C{{a}_{2}}{{B}_{6}}{{O}_{11}}\,.\,5{{H}_{2}}O\]
done
clear
C)
\[NaB{{O}_{2}}\]
done
clear
D)
\[{{H}_{3}}B{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 71) Arsenic drugs are mainly used in the treatment of
A)
jaundice
done
clear
B)
typhoid
done
clear
C)
syphilis
done
clear
D)
cholera
done
clear
View Answer play_arrow
question_answer 72) Which of the following statements is not correct?
A)
Methylamine is more basic than \[N{{H}_{3}}\]
done
clear
B)
Amines form hydrogen bonds
done
clear
C)
Ethylamine has higher boiling point than propane
done
clear
D)
Dimethylamine is less basic than methylamine
done
clear
View Answer play_arrow
question_answer 73) Which one of the following will be most basic?
A)
Aniline
done
clear
B)
p-methoxyaniline
done
clear
C)
p-nitroaniline
done
clear
D)
Benzylamine
done
clear
View Answer play_arrow
question_answer 74) The final product of oxidation of 2-propanol with hot cone. \[HN{{O}_{3}}\] is
A)
propanal
done
clear
B)
ethanoic acid
done
clear
C)
propanone
done
clear
D)
ethanol
done
clear
View Answer play_arrow
question_answer 75) Bimolecular reduction of acetone gives
A)
diacetoneamine
done
clear
B)
pinacol
done
clear
C)
chloretone
done
clear
D)
propane
done
clear
View Answer play_arrow
question_answer 76) Aldehydes and ketones give addition reaction with
A)
hydrazine
done
clear
B)
phenyl hydrazine
done
clear
C)
semicarbazide
done
clear
D)
hydrogen cyanide
done
clear
View Answer play_arrow
question_answer 77) Amongst the following, the compound which is most difficult to sulphonate is
A)
benzene
done
clear
B)
nitrobenzene
done
clear
C)
toluene
done
clear
D)
chlorobenzene
done
clear
View Answer play_arrow
question_answer 78) Acetonitrile is prepared by treating an alcoholic solution of methyl iodide with
A)
silver cyanide
done
clear
B)
potassium cyanide
done
clear
C)
hydrogen cyanide
done
clear
D)
ammonia
done
clear
View Answer play_arrow
question_answer 79) During polymerisation of acetylene to benzene, the state of hybridisation of-carbon changes from
A)
\[s{{p}^{2}}\]to sp
done
clear
B)
\[s{{p}^{3}}\] to sp
done
clear
C)
sp to \[s{{p}^{3}}\]
done
clear
D)
sp to \[s{{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 80) Which one of the following series contains electrophiles only?
A)
\[{{H}_{2}}O,\,S{{O}_{3}},{{H}_{3}}{{O}^{+}}\]
done
clear
B)
\[N{{H}_{3}},{{H}_{2}}O,\,AlC{{l}_{3}}\]
done
clear
C)
\[AlC{{l}_{3}},S{{O}_{3}},\overset{+}{\mathop{N}}\,{{O}_{2}}\]
done
clear
D)
\[{{H}_{2}}O,\overset{+}{\mathop{C}}\,l,\,N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 81) The equilibrium constants for the reactions \[{{N}_{2}}+3{{H}_{2}}2N{{H}_{3}}\] and \[\frac{1}{2}{{N}_{3}}+\frac{3}{2}{{H}_{2}}N{{H}_{3}}\] are \[{{K}_{1}}\] and \[{{K}_{2}}\] respectively. Which one of the following is the correct relationship?
A)
\[{{K}_{1}}=2{{K}_{2}}\]
done
clear
B)
\[{{K}_{1}}=\frac{1}{2}{{K}_{2}}\]
done
clear
C)
\[{{K}_{2}}=\sqrt{{{K}_{1}}}\]
done
clear
D)
\[{{K}_{1}}={{K}_{2}}\]
done
clear
View Answer play_arrow
question_answer 82) When we increase the temperature, the rate of reaction increases because of
A)
more number of collisions
done
clear
B)
decrease in mean free path
done
clear
C)
more number of energetic electrons
done
clear
D)
less number of energetic electrons
done
clear
View Answer play_arrow
question_answer 83) The pH of buffer of \[N{{H}_{4}}Cl\] type is given by
A)
\[pH=p{{K}_{b}}\]
done
clear
B)
\[pH=1/2p{{K}_{b}}-1/2\log \] [salt] /[base]
done
clear
C)
\[pH=14-p{{K}_{b}}-\log \] [salt]/[base]
done
clear
D)
\[pH=pOH-p{{K}_{b}}+\log \] [salt]/ [base]
done
clear
View Answer play_arrow
question_answer 84) Azimuthal quantum number determines the
A)
size
done
clear
B)
spin
done
clear
C)
orientation
done
clear
D)
angular momentum of orbitals
done
clear
View Answer play_arrow
question_answer 85) The formation of colloid from suspension is called
A)
peptisation
done
clear
B)
condensation
done
clear
C)
sedimentation
done
clear
D)
fragmentation
done
clear
View Answer play_arrow
question_answer 86) Other things being equal, the EMF of a Daniell cell may be increased by
A)
keeping low temperature
done
clear
B)
using large copper electrodes
done
clear
C)
using large zinc electrodes
done
clear
D)
decreasing concentration of \[C{{u}^{2+}}\] ions
done
clear
View Answer play_arrow
question_answer 87) Which one of the following can be considered as a weak electrolyte?
A)
\[NaCl\]
done
clear
B)
\[HCl\]
done
clear
C)
\[C{{H}_{3}}COOH\]
done
clear
D)
\[{{K}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 88) lodoform can be prepared from all except
A)
acetaldehyde
done
clear
B)
3-methyl-2-butanone
done
clear
C)
iso-butyl alcohol
done
clear
D)
acetophenone
done
clear
View Answer play_arrow
question_answer 89) The end product C in the following sequence of chemical reactions is \[C{{H}_{3}}COOH\xrightarrow{CaC{{O}_{3}}}A\xrightarrow{Heat}\xrightarrow{N{{H}_{2}}OH}C\]
A)
acetaldehyde oxime
done
clear
B)
formaldehyde oxime
done
clear
C)
methyl nitrate
done
clear
D)
acetoxime
done
clear
View Answer play_arrow
question_answer 90) Synthetic human hair wigs are made from a copolymer of vinyl chloride and acrylonitrile and is called
A)
PVC
done
clear
B)
polyacrylonitrile
done
clear
C)
cellulose
done
clear
D)
dynel
done
clear
View Answer play_arrow
question_answer 91) Which one of the following is not an example of chain growth polymer?
A)
Neoprene
done
clear
B)
Buna-S
done
clear
C)
PMMA
done
clear
D)
Glyptal
done
clear
View Answer play_arrow
question_answer 92) Most abundant water pollutant is
A)
detergents
done
clear
B)
industrial wastes
done
clear
C)
pesticides
done
clear
D)
oil spills
done
clear
View Answer play_arrow
question_answer 93) Paulings electronegativity values for elements are useful in predicting
A)
polarity of the molecules
done
clear
B)
position in the EMF series
done
clear
C)
coordination numbers
done
clear
D)
dipole moments
done
clear
View Answer play_arrow
question_answer 94) A neutral ferilizer among the following is
A)
CAN
done
clear
B)
ammonium sulphate
done
clear
C)
ammonium nitrate
done
clear
D)
urea
done
clear
View Answer play_arrow
question_answer 95) Transition metal with low oxidation number will act as
A)
an oxidising agent
done
clear
B)
abase
done
clear
C)
an acid
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 96) In \[LiAl{{H}_{4}}\], the ligand is
A)
H
done
clear
B)
\[{{H}^{+}}\]
done
clear
C)
\[{{H}^{-}}\]
done
clear
D)
\[2{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 97) Iodide of Millons base is
A)
\[Hg{{I}_{2}}\]
done
clear
B)
\[{{K}_{2}}Hg{{I}_{4}}\]
done
clear
C)
\[N{{H}_{2}}HgO\,.\,\,HgI\]
done
clear
D)
\[N{{H}_{4}}I\]
done
clear
View Answer play_arrow
question_answer 98) Ce-58 is a member of
A)
s-block elements
done
clear
B)
p-block elements
done
clear
C)
d-block elements
done
clear
D)
f-block elements
done
clear
View Answer play_arrow
question_answer 99) Complete hydrolysis of \[Xe{{F}_{2}}\] gives
A)
Xe
done
clear
B)
\[{{O}_{2}}\]
done
clear
C)
HF
done
clear
D)
AH of these
done
clear
View Answer play_arrow
question_answer 100) The alloy best suited for making metre scales is
A)
stainless steel
done
clear
B)
invar
done
clear
C)
alnico
done
clear
D)
tungsten steel
done
clear
View Answer play_arrow
question_answer 101) The capsid of virus is synthesized on
A)
nucleus of the virus
done
clear
B)
ribosomes of the host
done
clear
C)
mitochondria of the host
done
clear
D)
plasma membrane of the host
done
clear
View Answer play_arrow
question_answer 102) Which of the following is not a prorist?
A)
Amoeba
done
clear
B)
Taenia
done
clear
C)
Paramecium
done
clear
D)
Euglena
done
clear
View Answer play_arrow
question_answer 103) Archegonia are not found in
A)
bryophytes
done
clear
B)
pteridophytes
done
clear
C)
gymnosperms
done
clear
D)
angiosperms
done
clear
View Answer play_arrow
question_answer 104) Mycoplasma can multiply
A)
in culture media
done
clear
B)
in the body of living host
done
clear
C)
on dead and decaying matther
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 105) An intracellular parasite is
A)
Entamoeba
done
clear
B)
Ascaris
done
clear
C)
Trypanosoma
done
clear
D)
Plasmodium
done
clear
View Answer play_arrow
question_answer 106) Mycorrhizal association is must for growth of
A)
mushrooms
done
clear
B)
orchids
done
clear
C)
sal and teak
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 107) Antherozoids of pteridophyta are
A)
much coiled and multiciliated
done
clear
B)
pear-shaped and biflagellate
done
clear
C)
cork screw-shaped and multiflagellated
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 108) Pectin substance is generally stained with
A)
iodine
done
clear
B)
light green
done
clear
C)
cotton blue
done
clear
D)
ruthenium red
done
clear
View Answer play_arrow
question_answer 109) Blood cell which shows phagocytosis is
A)
eosinophyll
done
clear
B)
basophyll
done
clear
C)
monocyte
done
clear
D)
platelet
done
clear
View Answer play_arrow
question_answer 110) Amitosis is a division
A)
involving spindle formation
done
clear
B)
involving formation of chromosome bridges
done
clear
C)
in which chromosome number becomes half
done
clear
D)
in which cell divide
done
clear
View Answer play_arrow
question_answer 111) All of the followings are composed exclusively of glucose, except
A)
amylose
done
clear
B)
cellulose
done
clear
C)
lactose
done
clear
D)
maltose
done
clear
View Answer play_arrow
question_answer 112) The percentage of heterozygous individuals obtained from selfing Rr individual, is
A)
25
done
clear
B)
75
done
clear
C)
50
done
clear
D)
100
done
clear
View Answer play_arrow
question_answer 113) Balbiani rings are the centre for
A)
DNA synthesis
done
clear
B)
RNA synthesis
done
clear
C)
Both [a] and [b] synthesis
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 114) You are studying a bacterial virus and found its base composition to be A = 22%, T = 28%, G = 20% and C = 30%. What is your conclusion regarding its genetic material?
A)
Single-stranded RNA
done
clear
B)
Double-stranded RNA
done
clear
C)
Single-stranded DNA
done
clear
D)
Double-stranded DNA
done
clear
View Answer play_arrow
question_answer 115) Which one is the Pribnow box?
A)
5 TATAAT 3
done
clear
B)
5 TAATTA 3
done
clear
C)
5 AATAAT 3
done
clear
D)
5 ATATTA 3
done
clear
View Answer play_arrow
question_answer 116) A woman with Straight hair marries a man with curly hair who is known to be heterozygous for the trait. What is the chance that their first child will have curly hair?
A)
No chance
done
clear
B)
One in two
done
clear
C)
It is certain
done
clear
D)
One in four
done
clear
View Answer play_arrow
question_answer 117) Life originated in
A)
air
done
clear
B)
water
done
clear
C)
land
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 118) Which of these is mismatched?
A)
Coenozoic-Grasses and humans
done
clear
B)
Mesozoic -Cycads and dinosaurs
done
clear
C)
Palaeozoic-Prokaryotes and unicellular eukaryotes
done
clear
D)
Cambrian-Marine organism with external skeletons
done
clear
View Answer play_arrow
question_answer 119) 200 seeds are produced from how many fruits of maize?
A)
200
done
clear
B)
100
done
clear
C)
50
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 120) Outer part of bark consisting of dead cells refers to
A)
rhytidome
done
clear
B)
phellem
done
clear
C)
phellogen
done
clear
D)
phelloderm
done
clear
View Answer play_arrow
question_answer 121) A person supplied excess fertilizers and watered the plants well. After sometime, the leaves turn brown because
A)
exosmosis occurs in root and the plant dies
done
clear
B)
fertilizer is drained in lower layer of soil
done
clear
C)
it decreases photosynthesis
done
clear
D)
due to water logging soil
done
clear
View Answer play_arrow
question_answer 122) Which of the fallowing elements are used up in phosphorylaften?
A)
N and P
done
clear
B)
I and P
done
clear
C)
Ca and Mg
done
clear
D)
Ca and S
done
clear
View Answer play_arrow
question_answer 123) Agranal chloroplasts occur in certain
A)
succulents
done
clear
B)
\[{{C}_{4}}\]plants
done
clear
C)
hydrophytes
done
clear
D)
\[{{C}_{3}}\]plants
done
clear
View Answer play_arrow
question_answer 124) How many ATP and \[NAD{{H}_{2}}\] are produced in photorespiration?
A)
2 and 4
done
clear
B)
1 and 2
done
clear
C)
4 and 6
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 125) Embryonic connective tissue is derived from
A)
ectoderm
done
clear
B)
mesoderm
done
clear
C)
endoderm
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 126) Troponin is a
A)
muscle protein
done
clear
B)
digestive enzyme
done
clear
C)
water soluble vitamin
done
clear
D)
high energy reservoir
done
clear
View Answer play_arrow
question_answer 127) A renal portal system is found in
A)
man
done
clear
B)
frog
done
clear
C)
horse
done
clear
D)
rabbit
done
clear
View Answer play_arrow
question_answer 128) Amylopsin acts upon
A)
polypeptides
done
clear
B)
polysaccharide in any medium
done
clear
C)
polysaccharide in acidic medium
done
clear
D)
polysaccharide in alkaline medium
done
clear
View Answer play_arrow
question_answer 129) During forced expiration, actively contracting muscles include the
A)
diaphragm
done
clear
B)
external intercostals
done
clear
C)
abdominal muscles
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 130) Which term does not apply to human heart?
A)
Pace-maker
done
clear
B)
Mitral valve
done
clear
C)
Neurogenic
done
clear
D)
Four-chambered
done
clear
View Answer play_arrow
question_answer 131) Synthesis of urea in liver takes place by
A)
nitrogen cycle
done
clear
B)
Krebs cycle
done
clear
C)
ornithine cycle
done
clear
D)
glycolysis
done
clear
View Answer play_arrow
question_answer 132) A disease associated with pain and swelling is
A)
arthritis
done
clear
B)
Pagets disease
done
clear
C)
glaucoma
done
clear
D)
Homers syndrome
done
clear
View Answer play_arrow
question_answer 133) Acetylcholine is
A)
neural messenger
done
clear
B)
antistress hormone
done
clear
C)
chemical messenger
done
clear
D)
chemical transmitter
done
clear
View Answer play_arrow
question_answer 134) Which of the following hormones is a modified amino acid?
A)
MSH
done
clear
B)
Oestrogen
done
clear
C)
Epinephrine
done
clear
D)
Prostaglandin
done
clear
View Answer play_arrow
question_answer 135) Cybrids carry
A)
two similar genomes
done
clear
B)
only one genome
done
clear
C)
several genomes
done
clear
D)
one genome and two plasmones
done
clear
View Answer play_arrow
question_answer 136) Maximum amount of growth in root occurs
A)
in the absence of light
done
clear
B)
at its apex
done
clear
C)
behind the apex
done
clear
D)
in the presence of soil
done
clear
View Answer play_arrow
question_answer 137) Which statement about oocytes is true?
A)
At the onset of menopause, the woman stops producing them
done
clear
B)
They are produced by the human female throughout adiosecence
done
clear
C)
Those produced by the females are stored in the seminiferous tubules
done
clear
D)
At birth, the human female has produced all the oocytes she will ever produce
done
clear
View Answer play_arrow
question_answer 138) In which type of egg, total cleavage is possible?
A)
Telolecithal
done
clear
B)
Oligolecithal
done
clear
C)
Centrolecithal
done
clear
D)
Macrolecithal
done
clear
View Answer play_arrow
question_answer 139) Programmed cell death is scientifically termed as
A)
cell lysis
done
clear
B)
apoptosis
done
clear
C)
autotomy
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 140) Alluvial soil is a type of
A)
residual soil
done
clear
B)
transport soil
done
clear
C)
well mature soil
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 141) The extreme top of Mount Everest is in the region of
A)
stratosphere
done
clear
B)
stratopause
done
clear
C)
troposphere
done
clear
D)
mesosphere
done
clear
View Answer play_arrow
question_answer 142) A virgin ecosystem can be seen in
A)
Eastern Himalaya
done
clear
B)
Shimla
done
clear
C)
Nainital
done
clear
D)
Silent valley of Kerala
done
clear
View Answer play_arrow
question_answer 143) Transitional zone between two vegetational type is referred to
A)
ecotone
done
clear
B)
ecotype
done
clear
C)
ecades
done
clear
D)
ecophenes
done
clear
View Answer play_arrow
question_answer 144) Sewage water turns black due to action of
A)
\[{{H}_{2}}S\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[C{{H}_{4}}\]
done
clear
D)
\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 145) Which habitat shows highest diversity of living species?
A)
Deserts
done
clear
B)
Temperate forests
done
clear
C)
Tropical rainforests
done
clear
D)
Grasslands
done
clear
View Answer play_arrow
question_answer 146) AZT is the treatment of
A)
TB
done
clear
B)
AIDS
done
clear
C)
malaria
done
clear
D)
kala-azar
done
clear
View Answer play_arrow
question_answer 147) A disease caused by one of the smallest viruses is
A)
trachoma
done
clear
B)
poliomyelitis
done
clear
C)
mumps
done
clear
D)
measles
done
clear
View Answer play_arrow
question_answer 148) Tobacco fcdntains
A)
codeine
done
clear
B)
nicotine
done
clear
C)
morphine
done
clear
D)
cocaine
done
clear
View Answer play_arrow
question_answer 149) Membrane attack complex is formed by
A)
e-lymphocytes
done
clear
B)
macrophages
done
clear
C)
t-lymphocytes
done
clear
D)
complements
done
clear
View Answer play_arrow
question_answer 150) Axenic culture mean?
A)
cell culture
done
clear
B)
cell culture free from microorganisms
done
clear
C)
cell culture free from nutrients
done
clear
D)
None of the above
done
clear
View Answer play_arrow