question_answer 1) The height y and distance x along the horizontal plane of projectile on a certain planet (with no surrounding) are given by: \[y=(8t-5{{t}^{2}})\] metre and \[x=6\,t\] metre where r is in second. The velocity with which the projectile is projected is:
A)
8 m/s
done
clear
B)
6 m/s
done
clear
C)
10 m/s
done
clear
D)
data is not sufficient
done
clear
View Answer play_arrow
question_answer 2) A body of mass a, moving with a velocity b collides with a body of mass c, at rest and sticks to it. They move together with a velocity given by:
A)
\[\frac{ac}{a+b}\]
done
clear
B)
\[\frac{ab}{a+c}\]
done
clear
C)
\[\frac{a+b}{ac}\]
done
clear
D)
\[\frac{b+c}{ab}\]
done
clear
View Answer play_arrow
question_answer 3) The refractive index of a material is given by the equation \[n=\frac{A+B}{{{\lambda }^{2}}}\], where A and B are constants. The dimensional formula for B is:
A)
\[[{{M}^{0}}{{L}^{2}}{{T}^{-1}}]\]
done
clear
B)
\[[{{M}^{0}}{{L}^{-2}}{{T}^{0}}]\]
done
clear
C)
\[[{{M}^{0}}{{L}^{2}}{{T}^{-2}}]\]
done
clear
D)
\[[{{M}^{0}}{{L}^{0}}{{T}^{0}}]\]
done
clear
View Answer play_arrow
question_answer 4) A satellite is orbiting around the earth. By what percentage should we increase its velocity, so as to enable it escape away from the earth?
A)
41.4%
done
clear
B)
50%
done
clear
C)
82.8%
done
clear
D)
100%
done
clear
View Answer play_arrow
question_answer 5) At what temperature, the hydrogen molecule will escape from earths surface?
A)
101 K
done
clear
B)
102 K
done
clear
C)
103 K
done
clear
D)
104 K
done
clear
View Answer play_arrow
question_answer 6) If the earth is at one-fourth of its present. distance from the sun, the duration of the year will be :
A)
half the present year
done
clear
B)
one-eighth the present year
done
clear
C)
one-fourth the present year
done
clear
D)
one-sixth the present year
done
clear
View Answer play_arrow
question_answer 7) An observer moves towards a stationary source of sound with a velocity one-tenth the velocity of sound. The apparent increase in frequency is:
A)
zero
done
clear
B)
10%
done
clear
C)
5%
done
clear
D)
0.1%
done
clear
View Answer play_arrow
question_answer 8) When two conductors of charges and potentials \[{{C}_{1}},\,\,{{V}_{1}}\] and \[{{C}_{2}},\,\,{{V}_{2}}\] respectively are joined, the common potential will be :
A)
\[\frac{{{C}_{1}}{{V}_{1}}+{{C}_{2}}{{V}_{2}}}{{{V}_{1}}+{{V}_{2}}}\]
done
clear
B)
\[\frac{{{C}_{1}}V_{1}^{2}+{{C}_{2}}V_{2}^{2}}{V_{1}^{2}+V_{2}^{2}}\]
done
clear
C)
\[{{C}_{1}}+{{C}_{2}}\]
done
clear
D)
\[\frac{{{C}_{1}}{{V}_{1}}+{{C}_{2}}{{V}_{2}}}{{{C}_{1}}+{{C}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 9) A weightless thread can bear tension upto 3.7 kg-wt. A stone of mass 500 g is tied to it and revolved in a circular path of radius 4 m in a vertical plane. If \[g=10\,\,m{{s}^{-2}}\], then the maximum angular velocity of the stone will be:
A)
4 rad/s
done
clear
B)
16 rad/s
done
clear
C)
V21 rad/s
done
clear
D)
2 rad/s
done
clear
View Answer play_arrow
question_answer 10) The effective length of a magnet is 31.4 cm and its pole strength is 0.5 Am. If it is bent in the form of semicircle, what will be its magnetic moment then?
A)
0.12 \[A{{m}^{2}}\]
done
clear
B)
0.1 \[A{{m}^{2}}\]
done
clear
C)
0.05 \[A{{m}^{2}}\]
done
clear
D)
0.01 \[A{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 11) Four molecules of a gas have speeds 1, 2, 3 and\[4\,\,km{{s}^{-1}}\]. The value of rms speed of the gas molecules is:
A)
\[\frac{1}{2}\sqrt{15}\,km{{s}^{-1}}\]
done
clear
B)
\[\frac{1}{2}\sqrt{10}\,km{{s}^{-1}}\]
done
clear
C)
\[2.5\,\,km{{s}^{-1}}\]
done
clear
D)
\[\sqrt{\frac{15}{2}}\,\,km{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 12) If there is change of angular momentum from to 5 J in 5 s, then the torque is :
A)
\[\frac{3\,J}{5}\]
done
clear
B)
\[\frac{4\,J}{5}\]
done
clear
C)
\[\frac{5\,J}{4}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 13)
Two springs having force constants k each are arranged in parallel and in series. A mass M is attached to two arrangements separately. If time period in first case is \[{{T}_{1}}\] and in second case is \[{{T}_{2}}\], then ratio \[\frac{{{T}_{1}}}{{{T}_{2}}}\] is :
A)
1.5
done
clear
B)
3.2
done
clear
C)
0.5
done
clear
D)
2.1
done
clear
View Answer play_arrow
question_answer 14) If the work done in blowing a bubble of volume V is W, then the work done in blowing a soap bubble of volume 2 V will be :
A)
W
done
clear
B)
2W
done
clear
C)
V21V
done
clear
D)
\[{{4}^{1/3}}\] W
done
clear
View Answer play_arrow
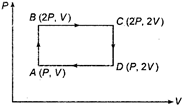
question_answer 15)
An ideal monoatomic gas is taken round the cycle ABCDA as shown in figure. The work done during the cycle is:
A)
PV
done
clear
B)
2PV
done
clear
C)
\[\frac{PV}{2}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 16) A proton of energy 2 MeV is moving in a circular path in a magnetic field. What should be the energy of a deuteron, so that it also describes circular path of radius equal to that of the proton?
A)
1 MeV
done
clear
B)
2 MeV
done
clear
C)
4 MeV
done
clear
D)
0.5 MeV
done
clear
View Answer play_arrow
question_answer 17) A gas at NTP is suddenly compressed to one-fourth of its original volume. If \[\gamma \] is supposed to be 3/2, then the final pressure is :
A)
4 arm
done
clear
B)
\[\frac{3}{2}\]arm
done
clear
C)
8 aim
done
clear
D)
\[\frac{1}{4}\]arm
done
clear
View Answer play_arrow
question_answer 18) In a series combination \[R=300\,\,\Omega \], L = 0.9 H, \[C=2.0\,\,\mu F,\,\,\omega =1000\] rad/s, the impedance of the circuit is :
A)
\[1300\,\,\Omega \]
done
clear
B)
\[900\,\,\Omega \]
done
clear
C)
\[500\,\,\Omega \]
done
clear
D)
\[400\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 19) n identical spherical drops each of radius r are charged to same potential V. They combine to form a bigger drop. The potential of the big drop will be :
A)
\[{{n}^{1/3}}V\]
done
clear
B)
\[{{n}^{2/3}}V\]
done
clear
C)
V
done
clear
D)
nV
done
clear
View Answer play_arrow
question_answer 20) The wavelength of maximum energy, released during an atomic explosion was \[2.9\times {{10}^{-10}}m\]. Given that the Wiens constant is \[2.93\times {{10}^{-10}}m\], the maximum temperature attained must be of the order of:
A)
\[{{10}^{-7}}K\]
done
clear
B)
\[{{10}^{7}}K\]
done
clear
C)
\[{{10}^{-3}}K\]
done
clear
D)
\[5.86\times {{10}^{-7}}K\]
done
clear
View Answer play_arrow
question_answer 21) The pressure and density of a diatomic gas \[\left( \gamma =\frac{7}{5} \right)\] change adiabatically from (P, d) to (P, d). If \[\frac{d}{d}=32\], then \[\frac{p}{p}\] should be :
A)
\[\frac{1}{128}\]
done
clear
B)
32
done
clear
C)
128
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 22) A piece of wax weighs 18.03 g in air. A piece of metal is found to weigh 17.03 g in water. It is tied to the wax and both together weigh 15.23 g in water. Then, the specific gravity of wax is :
A)
\[\frac{18.03}{17.03}\]
done
clear
B)
\[\frac{17.03}{18.03}\]
done
clear
C)
\[\frac{18.03}{19.83}\]
done
clear
D)
\[\frac{15.03}{17.03}\]
done
clear
View Answer play_arrow
question_answer 23) If a mica sheet of thickness t and refractive index u is placed in the path of one of interfering beams in a double slit experiment, then displacement of fringes will be :
A)
\[\frac{D}{d}\mu t\]
done
clear
B)
\[\frac{D}{d}(\mu -1)\,t\]
done
clear
C)
\[\frac{D}{d}(\mu +1)\,t\]
done
clear
D)
\[\frac{D}{d}({{\mu }^{2}}-1)\,t\]
done
clear
View Answer play_arrow
question_answer 24) A ray of light propagates from glass (refractive index \[=\frac{3}{2}\]) to water (refractive index \[=\frac{4}{3}\]). The value of the critical angle is :
A)
\[{{\sin }^{-1}}\left( \frac{1}{2} \right)\]
done
clear
B)
\[{{\sin }^{-1}}\left( \sqrt{\frac{9}{8}} \right)\]
done
clear
C)
\[{{\sin }^{-1}}\left( \frac{8}{9} \right)\]
done
clear
D)
\[{{\sin }^{-1}}\left( \frac{5}{7} \right)\]
done
clear
View Answer play_arrow
question_answer 25) A ray of light suffers minimum deviation when incident at \[{{60}^{o}}\] prism of refractive index \[\sqrt{2}\]. The angle of incidence is :
A)
\[{{\sin }^{-1}}(0.8)\]
done
clear
B)
\[{{60}^{o}}\]
done
clear
C)
\[{{45}^{o}}\]
done
clear
D)
\[{{30}^{o}}\]
done
clear
View Answer play_arrow
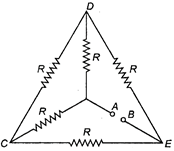
question_answer 26)
Each of the resistance in the network shown in figure is equal to R. Find the equivalent resistance between two terminals A and B.
A)
R
done
clear
B)
SR
done
clear
C)
2R
done
clear
D)
-JR
done
clear
View Answer play_arrow
question_answer 27) A gas in an air tight container is heated from \[{{25}^{o}}C\] to \[{{90}^{o}}C\]. The density of gas will:
A)
increase slightly
done
clear
B)
remain the same
done
clear
C)
increase considerably
done
clear
D)
decrease slightly
done
clear
View Answer play_arrow
question_answer 28) If 2% of the main current is to be passed through the galvanometer of resistance G, the resistance of the shunt required is :
A)
\[\frac{G}{49}\]
done
clear
B)
\[\frac{G}{50}\]
done
clear
C)
49 G
done
clear
D)
50 G
done
clear
View Answer play_arrow
question_answer 29) The current in self-inductance L = 40 mH is increased uniformly from 1 A to 11 A in 4 milliseconds. The induced emf produced in L during this process will be :
A)
100 V
done
clear
B)
0.2V
done
clear
C)
440V
done
clear
D)
40V
done
clear
View Answer play_arrow
question_answer 30) \[{{H}^{+}},H{{e}^{2+}}\] and \[{{O}^{2-}}\] all having the same kinetic energy pass through a region in which there is a uniform magnetic field perpendicular to their velocity. The masses of \[{{H}^{+}},H{{e}^{2+}}\] and \[{{O}^{2-}}\] are 1 amu, 4 amu and 16 amu, respectively. Then:
A)
\[{{H}^{+}}\] will be deflected most
done
clear
B)
\[{{O}^{2-}}\]will be deflected most
done
clear
C)
\[H{{e}^{2+}}\] and \[{{O}^{2-}}\] will be deflected most
done
clear
D)
all will be deflected most
done
clear
View Answer play_arrow
question_answer 31) The current gain of a transistor in common emitter mode is 49. The change in collector current and emitter current corresponding to the change in base current by 5.0 \[\mu A\] are :
A)
\[\Delta {{i}_{C}}=245\,\mu A,\,\Delta {{i}_{E}}=250\,\mu A\]
done
clear
B)
\[\Delta {{i}_{C}}=252\,\mu A,\,\Delta {{i}_{E}}=145\,\mu A\]
done
clear
C)
\[\Delta {{i}_{C}}=125\,\mu A,\,\Delta {{i}_{E}}=250\,\mu A\]
done
clear
D)
\[\Delta {{i}_{C}}=252\,\mu A,\,\Delta {{i}_{E}}=230\,\mu A\]
done
clear
View Answer play_arrow
question_answer 32) In hydrogen atom when an electron jumps from second to first orbit, the wavelength of line emitted is :
A)
0.563 \[\overset{o}{\mathop{A}}\,\]
done
clear
B)
4861 \[\overset{o}{\mathop{A}}\,\]
done
clear
C)
4102 \[\overset{o}{\mathop{A}}\,\]
done
clear
D)
1213 \[\overset{o}{\mathop{A}}\,\]
done
clear
View Answer play_arrow
question_answer 33) How does the magnetic susceptibility y, of a paramagnetic material change with absolute temperature T?
A)
\[\chi \propto T\]
done
clear
B)
\[\chi \propto {{T}^{-1}}\]
done
clear
C)
\[\chi =\]constant
done
clear
D)
\[\chi ={{e}^{T}}\]
done
clear
View Answer play_arrow
question_answer 34) Two identical heaters of 220 V, 1000 W are placed in parallel with each other across 220 V line, then the combined power is :
A)
1000 W
done
clear
B)
2000 W
done
clear
C)
500 W
done
clear
D)
4000 W
done
clear
View Answer play_arrow
question_answer 35) A bar of magnetic moment M is cut into two parts of equal length. The magnetic moment of either part is :
A)
M
done
clear
B)
2M
done
clear
C)
\[\frac{M}{2}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 36) A rain drop of radius 0.3 mm has a terminal velocity of 1 m/s and the viscosity of 1 m/s and the viscosity of air is \[18\times {{10}^{-5}}\] poise. The viscous force on the drop is :
A)
\[16.95\times {{10}^{-9}}N\]
done
clear
B)
\[1.695\times {{10}^{-9}}N\]
done
clear
C)
\[10.17\times {{10}^{-9}}N\]
done
clear
D)
\[101.74\times {{10}^{-9}}N\]
done
clear
View Answer play_arrow
question_answer 37) If magnetic material-moves from stronger to weaker parts of magnetic field, then it is known as :
A)
anti-ferromagnetic
done
clear
B)
ferromagnetic
done
clear
C)
diamagnetic
done
clear
D)
paramagnetic
done
clear
View Answer play_arrow
question_answer 38) A charge q is placed at the centre of line joining two equal charges Q. The system of three charges will be in equilibrium, if q is equal to:
A)
\[-\frac{Q}{2}\]
done
clear
B)
\[-\frac{Q}{4}\]
done
clear
C)
\[+\frac{Q}{4}\]
done
clear
D)
\[+\frac{Q}{2}\]
done
clear
View Answer play_arrow
question_answer 39) The temperature of cold, hot junction of a thermocouple are \[{{0}^{o}}C\] and \[{{T}^{o}}C\] respectively. The thermo-emf produced is \[E=AT-\frac{1}{2}B{{T}^{2}}\]. If A = 16, B = 0.08, the temperature of inversion will be:
A)
\[{{100}^{o}}C\]
done
clear
B)
\[{{300}^{o}}C\]
done
clear
C)
\[{{400}^{o}}C\]
done
clear
D)
\[{{500}^{o}}C\]
done
clear
View Answer play_arrow
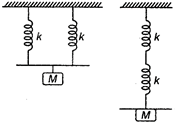
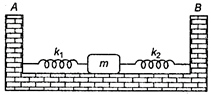
question_answer 40)
Two light springs of force constants \[{{k}_{1}}\] and \[{{k}_{2}}\]and a block of mass m are in one line AB on a smooth horizontal table, such that one end of each spring is fixed to rigid support and other end is attached to block of mass m kg as shown in figure. The frequency of vibration is :
A)
\[n=\frac{1}{2\pi }\sqrt{\frac{{{k}_{1}}+{{k}_{2}}}{m}}\]
done
clear
B)
\[n=\frac{1}{2\pi }\sqrt{\frac{{{k}_{1}}\,{{k}_{2}}}{m}}\]
done
clear
C)
\[n=\frac{1}{2\pi }\sqrt{\frac{{{k}_{1}}-{{k}_{2}}}{m}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 41) Pressure inside two soap bubbles are 1.01 and 1.02 atm. Ratio between their volumes is :
A)
102 : 101
done
clear
B)
\[{{(102)}^{3}}\text{:(}103{{)}^{3}}\]
done
clear
C)
8 : 1
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 42)
Two dielectrics of dielectric constants \[{{K}_{1}}\] and \[{{K}_{2}}\] are filled in gap of parallel plate capacitor as shown in figure
A)
\[\frac{{{\varepsilon }_{0}}A\,({{K}_{1}}+{{K}_{2}})}{2\,d}\]
done
clear
B)
\[\frac{{{\varepsilon }_{0}}A}{2\,d}\left( \frac{({{K}_{1}}+{{K}_{2}})}{{{K}_{1}}{{K}_{2}}} \right)\]
done
clear
C)
\[\frac{{{\varepsilon }_{0}}}{d}\left( \frac{({{K}_{1}}{{K}_{2}}}{{{K}_{1}}+{{K}_{2}})} \right)\]
done
clear
D)
\[\frac{{{\varepsilon }_{0}}A}{d}\left( \frac{({{K}_{1}}+{{K}_{2}})}{{{K}_{1}}{{K}_{2}}} \right)\]
done
clear
View Answer play_arrow
question_answer 43) For a series LCR circuit, the phase difference between current and voltage at the condition of resonance will be :
A)
\[\frac{\pi }{2}\]
done
clear
B)
\[\frac{\pi }{4}\]
done
clear
C)
zero
done
clear
D)
nothing can be said
done
clear
View Answer play_arrow
question_answer 44) A metallic rod of length \[l\] is placed normal to the magnetic field B and revolved in a circular path about one of the ends with angular frequency co. The potential difference across the ends will be ;
A)
\[\frac{1}{2}{{B}^{2}}l\omega \]
done
clear
B)
\[\frac{1}{2}B\omega {{l}^{2}}\]
done
clear
C)
\[\frac{1}{8}B\omega {{l}^{3}}\]
done
clear
D)
\[B\omega {{l}^{2}}\]
done
clear
View Answer play_arrow
question_answer 45) A magnetic needle suspended in a vertical plane at \[{{30}^{o}}\] from the magnetic meridian makes an angle \[{{45}^{o}}\] with the horizontal. What will be the true angle of dip?
A)
\[{{\tan }^{-1}}\left( \frac{\sqrt{3}}{2} \right)\]
done
clear
B)
\[{{\tan }^{-1}}(\sqrt{3})\]
done
clear
C)
\[{{45}^{o}}\]
done
clear
D)
\[{{30}^{o}}\]
done
clear
View Answer play_arrow
question_answer 46) A force F is given by \[F=at+b{{t}^{2}}\], where t is time. What are the dimensions of a and b respectively?
A)
\[[ML{{T}^{-1}}]\] and \[[ML{{T}^{-4}}]\]
done
clear
B)
\[[ML{{T}^{-3}}]\] and \[[ML{{T}^{-4}}]\]
done
clear
C)
\[[ML{{T}^{-4}}]\] and \[[ML{{T}^{2}}]\]
done
clear
D)
\[[M{{L}^{2}}{{T}^{3}}]\] and \[[M{{L}^{-1}}{{T}^{2}}]\]
done
clear
View Answer play_arrow
question_answer 47) In a triode valve, the plate resistance is \[10000\,\,\Omega \] and the anode load resistance is\[30000\,\,\Omega \]. If the amplification factor is 36, then the voltage gain is :
A)
9
done
clear
B)
27
done
clear
C)
36
done
clear
D)
108
done
clear
View Answer play_arrow
question_answer 48) \[{{g}_{e}}\] and \[{{g}_{p}}\] denote the acceleration due to gravity on the surface of the earth and another planet whose mass and radius are twice to that of the earth, then :
A)
\[{{g}_{p}}=\frac{{{g}_{e}}}{2}\]
done
clear
B)
\[{{g}_{p}}={{g}_{e}}\]
done
clear
C)
\[{{g}_{p}}=2{{g}_{e}}\]
done
clear
D)
\[{{g}_{p}}=\frac{{{g}_{e}}}{\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 49) Of the following which relation is true :
A)
\[\beta >\alpha \]
done
clear
B)
\[\alpha >\beta \]
done
clear
C)
\[\alpha \beta =1\]
done
clear
D)
\[\alpha =\beta \]
done
clear
View Answer play_arrow
question_answer 50) A soap bubble in vacuum has a radius 3 cm and another soap bubble in vacuum has radius 4 cm. If two bubbles coalesce under isothermal condition, then the radius of the new bubble will be :
A)
7 cm
done
clear
B)
5 cm
done
clear
C)
4.5 cm
done
clear
D)
2.3 cm
done
clear
View Answer play_arrow
question_answer 51) Out of Cu, Al Fe and Zn, metal which can displace all others from their salt solution is:
A)
Al
done
clear
B)
Cu
done
clear
C)
Zn
done
clear
D)
Fe
done
clear
View Answer play_arrow
question_answer 52) In which of the following reactions, hydrogen is acting as an oxidising agent?
A)
With \[Li\] to form \[LiH\]
done
clear
B)
With \[{{I}_{2}}\] to give HI
done
clear
C)
With S to give \[{{H}_{2}}S\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 53) Our cells get energy by the conversion of:
A)
ATP \[\xrightarrow{{}}\] Adenine
done
clear
B)
ATP \[\xrightarrow{{}}\] ADP
done
clear
C)
ADP \[\xrightarrow{{}}\] AMP
done
clear
D)
CDP \[\xrightarrow{{}}\] CTP
done
clear
View Answer play_arrow
question_answer 54) The number of optically active isomers of tartaric acid are:
A)
1
done
clear
B)
3
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 55) The hardness of water is estimated by :
A)
conductivity method
done
clear
B)
titrimetric method
done
clear
C)
EDTA method
done
clear
D)
distillation method
done
clear
View Answer play_arrow
question_answer 56) \[N{{a}_{2}}O,MgO,\,\,A{{l}_{2}}{{O}_{3}}\] and \[Si{{O}_{2}}\] have heat of formation equal to -416, -602, -1676 and \[-911\,\,kJ\,\,mo{{l}^{-1}}\] respectively. The most stable oxide is :
A)
\[N{{a}_{2}}O\]
done
clear
B)
\[MgO\]
done
clear
C)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
D)
\[Si{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 57) A photon having a wavelength of \[845\text{ }\overset{o}{\mathop{A}}\,\], causes the ionisation of N atom. What is the ionization energy of N?
A)
1.4 kJ
done
clear
B)
\[1.4\times {{10}^{4}}\,kJ\]
done
clear
C)
\[1.4\times {{10}^{2}}\,kJ\]
done
clear
D)
\[1.4\times {{10}^{3}}\,kJ\]
done
clear
View Answer play_arrow
question_answer 58) The electronic theory of bonding was proposed by:
A)
Pauling
done
clear
B)
Lewis
done
clear
C)
Bronsted
done
clear
D)
Mullikan
done
clear
View Answer play_arrow
question_answer 59) An azeotropic mixture of two liquids has boiling point lower than either of them, when it:
A)
shows a negative deviation from Raoults law
done
clear
B)
shows no deviation from Raoults law
done
clear
C)
shows positive deviation from Raoults law
done
clear
D)
is saturated
done
clear
View Answer play_arrow
question_answer 60) Troutons rule gives the relation between:
A)
\[{{T}_{b}}\] and T,
done
clear
B)
\[{{T}_{b}}\] and critical pressure
done
clear
C)
enthalpy of vaporisation and boiling point
done
clear
D)
normal boiling point and boiling point
done
clear
View Answer play_arrow
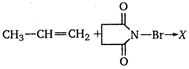
question_answer 61)
The product \[X\] in the following reaction is:
A)
\[C{{H}_{2}}Br-CH=C{{H}_{2}}\]
done
clear
B)
\[C{{H}_{2}}-\overset{\begin{smallmatrix} Br \\ | \end{smallmatrix}}{\mathop{C}}\,=C{{H}_{2}}\]
done
clear
C)
\[C{{H}_{3}}CH=CHBr\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 62) What are the products in the following reaction? \[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}\xrightarrow[cold]{HI}X+Y\]
A)
\[C{{H}_{3}}COOH,\,\,C{{H}_{2}}=C{{H}_{2}}\]
done
clear
B)
\[C{{H}_{3}}CHO,C{{H}_{2}}=C{{H}_{2}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}OH,{{C}_{2}}{{H}_{5}}I\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 63) Which of the following reagent can distinguish between butyne-1 and butyne-2?
A)
Bromine water
done
clear
B)
Aqueous \[NaOH\]
done
clear
C)
Fehlings solution
done
clear
D)
Ammoniacal \[AgN{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 64) Which of the following will have maximum pH?
A)
\[\frac{M}{10}HCl\]
done
clear
B)
\[\frac{M}{100}HCl\]
done
clear
C)
\[\frac{M}{10}NaOH\]
done
clear
D)
\[\frac{M}{100}NaOH\]
done
clear
View Answer play_arrow
question_answer 65) What is \[\Delta E\] for system that does 500 cal of work on surrounding and 300 cal of heat is absorbed by the system?
A)
-200 cal
done
clear
B)
-300 cal
done
clear
C)
+200 cal
done
clear
D)
+300 cal
done
clear
View Answer play_arrow
question_answer 66) The rate of a chemical reaction :
A)
increase as the reaction proceeds
done
clear
B)
decrease as the reaction proceeds
done
clear
C)
may increase or decrease during reaction
done
clear
D)
remain constant as the reaction
done
clear
View Answer play_arrow
question_answer 67) A carbonate ore is :
A)
carnallite
done
clear
B)
limonite
done
clear
C)
siderite
done
clear
D)
horn silver
done
clear
View Answer play_arrow
question_answer 68) Cadmium in a nuclear reactor acts as :
A)
nuclear fuel
done
clear
B)
neutron absorber
done
clear
C)
a moderator
done
clear
D)
neutron liberator to start the chain
done
clear
View Answer play_arrow
question_answer 69) Cement does not contain :
A)
calcium
done
clear
B)
aluminium
done
clear
C)
sulphur
done
clear
D)
iron
done
clear
View Answer play_arrow
question_answer 70) The simplest way, to check whether a system is a colloid, is by :
A)
Tyndall effect
done
clear
B)
Brownian movement
done
clear
C)
Electrodialysis
done
clear
D)
finding out particle size
done
clear
View Answer play_arrow
question_answer 71)
What is Z in the following reaction?
A)
Benzoic acid
done
clear
B)
Cyanobenzoic acid
done
clear
C)
Benzamide
done
clear
D)
Aniline
done
clear
View Answer play_arrow
question_answer 72) The reaction, \[RCOOH\xrightarrow{Na{{N}_{3}}/conc.\,\,{{H}_{2}}S{{O}_{4}}}\] \[RN{{H}_{2}}+{{N}_{2}}+C{{O}_{2}}\] is known as :
A)
Curtius reaction
done
clear
B)
Lossen reaction
done
clear
C)
Schmidt reaction
done
clear
D)
Hofmann reaction
done
clear
View Answer play_arrow
question_answer 73) What is the product in the reaction? \[C{{H}_{3}}MgBr\xrightarrow[(ii){{H}_{2}}O]{(i)C{{O}_{2}}}X\]
A)
Acetaldehyde
done
clear
B)
Acetic acid
done
clear
C)
Formic acid
done
clear
D)
Formaldehyde
done
clear
View Answer play_arrow
question_answer 74) What is the value of \[{{E}_{cell}}\]? \[Cr|C{{r}^{3+}}(0.1M)|\,\,|F{{e}^{2+}}\,(0.01M)|Fe\] Given, \[E_{C{{r}^{3+}}/Cr}^{o}=-0.74\,V\]and \[E_{F{{e}^{2+}}/Fe}^{o}=-0.44\,V\]
A)
+0.29 41V
done
clear
B)
+ 0.5212 V
done
clear
C)
+ 0.1308V
done
clear
D)
-0.2606 V
done
clear
View Answer play_arrow
question_answer 75) Elevation in boiling point was \[{{0.52}^{o}}C\] when 6g of a compound was dissolved in 100 g of water. Molecular weight of X is (\[{{K}_{b}}\] of water is \[{{5.2}^{o}}C\]per 100 g of water):
A)
120
done
clear
B)
60
done
clear
C)
600
done
clear
D)
180
done
clear
View Answer play_arrow
question_answer 76) The solubility of \[PbC{{l}_{2}}\] is :
A)
\[\sqrt{{{K}_{sp}}}\]
done
clear
B)
\[{{({{K}_{sp}})}^{1/3}}\]
done
clear
C)
\[{{({{K}_{sp}}/4)}^{1/3}}\]
done
clear
D)
\[{{(8{{K}_{sp}})}^{1/2}}\]
done
clear
View Answer play_arrow
question_answer 77) Equilibrium constant K , for the reaction, \[2HI(g){{H}_{2}}(g)+{{I}_{2}}(g)\]at room temperature is 2.85 and that at 698 K is \[1.4\times {{10}^{-2}}\]. This implies that the forward reaction is:
A)
exothermic
done
clear
B)
endothermic
done
clear
C)
exergonic
done
clear
D)
unpredictable
done
clear
View Answer play_arrow
question_answer 78) Which of the following represents hexadentate ligand?
A)
2,2-bipyridyl
done
clear
B)
DMG
done
clear
C)
Ethylenediamine
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 79) The amount of substance that gives \[3.7\times {{10}^{7}}\]dps, is :
A)
one becquerel
done
clear
B)
one curie
done
clear
C)
one millicurie
done
clear
D)
one Rutherford
done
clear
View Answer play_arrow
question_answer 80) The reaction, \[A{{g}^{2+}}(aq)+Ag(s)2A{{g}^{+}}(aq)\] is an example of:
A)
reduction
done
clear
B)
oxidation
done
clear
C)
comproportionation
done
clear
D)
disproportionation
done
clear
View Answer play_arrow
question_answer 81) DDT is obtained by the reaction of chlorobenzene with:
A)
chloral
done
clear
B)
chloroform
done
clear
C)
dichloromethane
done
clear
D)
acetaldehyde
done
clear
View Answer play_arrow
question_answer 82) Which of the following is false?
A)
Glycerol has strong hydrogen bonding
done
clear
B)
Glycol is a poisonous alcohols
done
clear
C)
Waxes are esters of higher alcohols with higher acids
done
clear
D)
Alkyl halides have higher b.p. than corresponding alcohols
done
clear
View Answer play_arrow
question_answer 83) Which of the following oxides is most acidic?
A)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
B)
\[Si{{O}_{3}}\]
done
clear
C)
\[{{P}_{2}}{{O}_{5}}\]
done
clear
D)
\[MgO\]
done
clear
View Answer play_arrow
question_answer 84) Glaubers salt is :
A)
\[N{{a}_{2}}C{{O}_{3}}\,.\,\,10{{H}_{2}}O\]
done
clear
B)
\[N{{a}_{2}}S{{O}_{4}}\,.\,\,10{{H}_{2}}O\]
done
clear
C)
\[MgS{{O}_{4}}\,.\,\,7{{H}_{2}}O\]
done
clear
D)
\[CaS{{O}_{4}}\,.\,\,5{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 85) Enthalpy change when 1g water is frozen at\[{{0}^{o}}C\] is : (\[\Delta {{H}_{fus}}=1.435\] kcal \[mo{{l}^{-1}}\])
A)
0.0797 kcal
done
clear
B)
-0.0797 kcal
done
clear
C)
1.435 kcal
done
clear
D)
-1.435 kcal
done
clear
View Answer play_arrow
question_answer 86) Which of the following statement is true?
A)
Some complex metal oxides behave as superconductor
done
clear
B)
Zinc oxide can act as superconductor
done
clear
C)
An impurity of tetravalent germanium in trivalent gallium creates electron deficiency
done
clear
D)
A Frenkel defect is formed when an ion is displaced from its lattice site to an interstitial site
done
clear
View Answer play_arrow
question_answer 87) The correct set of four quantum number for the valence electron of rubidium (Z = 37) is :
A)
\[n=5,\,l=0,\,\,m=0,\,\,s=+1/2\]
done
clear
B)
\[n=5,\,l=1,\,\,m=1,\,\,s=+1/2\]
done
clear
C)
\[n=5,\,l=1,\,\,m=1,\,\,s=+1/2\]
done
clear
D)
\[n=6,\,l=0,\,\,m=0,\,\,s=+1/2\]
done
clear
View Answer play_arrow
question_answer 88) \[s{{p}^{3}}\]-hybridisation is not found in :
A)
\[{{H}_{2}}O\]
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
\[BC{{l}_{3}}\]
done
clear
D)
\[N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 89) Chalcopyrites is an ore of:
A)
gallium
done
clear
B)
copper
done
clear
C)
calcium
done
clear
D)
magnesium
done
clear
View Answer play_arrow
question_answer 90) Which of the following statement is correct?
A)
Acidify increases with increase in carbon atoms in carboxylic acids
done
clear
B)
Solubility of carboxylic acids increases with increase in carbon atoms
done
clear
C)
foiling points of acids are higher than corresponding alcohols
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 91) Which temperature is most suitable for fermentation?
A)
273 K
done
clear
B)
298 K
done
clear
C)
350 K
done
clear
D)
330 K
done
clear
View Answer play_arrow
question_answer 92) In the reaction, \[HCHO+N{{H}_{3}}\xrightarrow{{}}X,\,\,X\]is :
A)
meta-formaldehyde
done
clear
B)
para-formaldehyde
done
clear
C)
urotropine
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 93) Which of the following have highest melting points?
A)
p -block elements
done
clear
B)
s-block elements
done
clear
C)
d-block elements
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 94) An acid has pH = 5 and its concentration is 1M. What is the value of \[{{K}_{a}}\] for the acid?
A)
\[{{10}^{-7}}\]
done
clear
B)
\[{{10}^{-5}}\]
done
clear
C)
\[{{10}^{-10}}\]
done
clear
D)
\[{{10}^{-8}}\]
done
clear
View Answer play_arrow
question_answer 95) Fatty acid is to fat as glucose is to :
A)
cellulose
done
clear
B)
glycogen
done
clear
C)
starch
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 96) In aerosol, the dispersion medium is :
A)
solid
done
clear
B)
liquid
done
clear
C)
gas
done
clear
D)
any of these
done
clear
View Answer play_arrow
question_answer 97) Peptisation denotes :
A)
digestion of food
done
clear
B)
hydrolysis of protein
done
clear
C)
breaking and dispersion into colloidal state
done
clear
D)
precipitation of solid from colloidal state
done
clear
View Answer play_arrow
question_answer 98) Buna-N is a polymer of:
A)
butadiene and isoprene
done
clear
B)
butadiene and acrylonitrile
done
clear
C)
isoprene and ethylene diamine
done
clear
D)
isoprene and butyl diamine
done
clear
View Answer play_arrow
question_answer 99) Which of the following is an ingrain dye?
A)
Congo-red
done
clear
B)
Aniline black
done
clear
C)
Alizarin
done
clear
D)
Indigo
done
clear
View Answer play_arrow
question_answer 100) Which of the following give an explosive, RDX, on nitration?
A)
Toluene
done
clear
B)
Benzene
done
clear
C)
Guanidine
done
clear
D)
Urotropine
done
clear
View Answer play_arrow
question_answer 101) Hepatitis-B is also called :
A)
epidemic jaundice
done
clear
B)
serum jaundice
done
clear
C)
catarrhal jaundice
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 102) In mammals, carbohydrates are stored as :
A)
glycogen in liver and spleen
done
clear
B)
lactic acid in muscles
done
clear
C)
glucose in liver
done
clear
D)
glycogen in liver and muscles
done
clear
View Answer play_arrow
question_answer 103) Raffinose is:
A)
monosaccharides
done
clear
B)
disaccharides
done
clear
C)
trisaccharides
done
clear
D)
polysaccharides
done
clear
View Answer play_arrow
question_answer 104) During conversion of pyruvic acid into acetyl Co-A, pyruvic acid is :
A)
oxidised
done
clear
B)
reduced
done
clear
C)
isomerised
done
clear
D)
condensed
done
clear
View Answer play_arrow
question_answer 105) A cell coded protein formed in response to infection with most animal viruses is :
A)
histone
done
clear
B)
antigen
done
clear
C)
antibody
done
clear
D)
interferon
done
clear
View Answer play_arrow
question_answer 106) In Krebs cycle :
A)
ADP is converted into ATP
done
clear
B)
pyruvic acid is converted into \[C{{O}_{2}}\] and \[{{H}_{2}}O\]
done
clear
C)
glucose is converted into \[C{{O}_{2}}\]
done
clear
D)
pyruvic acid is converted into ATP
done
clear
View Answer play_arrow
question_answer 107) Active transport:
A)
releases energy
done
clear
B)
requires energy
done
clear
C)
produces ATP
done
clear
D)
produces a toxic substance
done
clear
View Answer play_arrow
question_answer 108) The first carbon-dioxide acceptor in \[{{C}_{4}}\] plants is :
A)
phosphoenol pyruvate
done
clear
B)
oxalo acetic acid
done
clear
C)
phosphoglyceric acid
done
clear
D)
ribulose 1, 5- diphosphate
done
clear
View Answer play_arrow
question_answer 109) Fatty liver syndrome is due to excessive intake of:
A)
morphine
done
clear
B)
alcohol
done
clear
C)
tobacco
done
clear
D)
both [b] and [c]
done
clear
View Answer play_arrow
question_answer 110) The \[{{C}_{4}}\] plants are different from the ?3 plants with reference to the :
A)
types of pigments involved in photosynthesis
done
clear
B)
the number of NADPH that are consumed in preparing sugar
done
clear
C)
types of end product of photosynthesis
done
clear
D)
the substance that accepts \[C{{O}_{2}}\] in carbon assimilation and first stable product
done
clear
View Answer play_arrow
question_answer 111) Haversian canal is found in the bone of :
A)
mammals
done
clear
B)
reptiles
done
clear
C)
aves
done
clear
D)
pices
done
clear
View Answer play_arrow
question_answer 112) The gestation period of elephant is about:
A)
11 months
done
clear
B)
15 months
done
clear
C)
22 months
done
clear
D)
32 months
done
clear
View Answer play_arrow
question_answer 113) The banding pattern of chromosomes 3 and 6 of human beings and chimpanzee shows that they had :
A)
common origin
done
clear
B)
different origin
done
clear
C)
same number of chromosomes
done
clear
D)
similar blood groups
done
clear
View Answer play_arrow
question_answer 114) Most radiosensitive tissue of body is :
A)
bone-marrow
done
clear
B)
platelet
done
clear
C)
nervous tissue
done
clear
D)
lymphocyte
done
clear
View Answer play_arrow
question_answer 115) Acid rain are produced by :
A)
excess release of carbon-monoxide by incomplete combustion
done
clear
B)
excess formation of \[C{{O}_{2}}\] by combustion and animal respiration
done
clear
C)
excess production of \[N{{H}_{3}}\] by industry and coal gas
done
clear
D)
excess \[N{{O}_{2}}\] and \[S{{O}_{2}}\] from burning fossil fuels
done
clear
View Answer play_arrow
question_answer 116) Spermatogenesis is influenced by :
A)
Progesterone
done
clear
B)
FSH
done
clear
C)
STH
done
clear
D)
LTH
done
clear
View Answer play_arrow
question_answer 117) DNA acts as a template for synthesis of:
A)
RNA
done
clear
B)
DNA
done
clear
C)
both [a] and [b]
done
clear
D)
protein
done
clear
View Answer play_arrow
question_answer 118) t-RNA attaches, amino acid at its :
A)
3end
done
clear
B)
5end
done
clear
C)
anticodon
done
clear
D)
loop
done
clear
View Answer play_arrow
question_answer 119) DPT provide immunity against:
A)
diphtheria
done
clear
B)
whooping cough
done
clear
C)
tetanus
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 120) External water is not required for fertilization of:
A)
bryophytes
done
clear
B)
pteridophytes
done
clear
C)
thallophytes
done
clear
D)
spermatophytes
done
clear
View Answer play_arrow
question_answer 121) In orthotropous ovule, the micropyle and chalaza are :
A)
oblique to funiculus
done
clear
B)
parallel to funiculus
done
clear
C)
at right angle to funiculus
done
clear
D)
in straight line with funiculus
done
clear
View Answer play_arrow
question_answer 122) In India AIDS was reported in :
A)
1932
done
clear
B)
1986
done
clear
C)
1990
done
clear
D)
1992
done
clear
View Answer play_arrow
question_answer 123) A colourblind person can not distinguish :
A)
green and blue
done
clear
B)
red and green
done
clear
C)
yellow and white
done
clear
D)
black and yellow
done
clear
View Answer play_arrow
question_answer 124) On which day, we celebrate malarial day?
A)
5th June
done
clear
B)
15th August
done
clear
C)
20th August
done
clear
D)
1st December
done
clear
View Answer play_arrow
question_answer 125) Proteinaceous nature of enzyme was suggested by:
A)
Sumner
done
clear
B)
Kuhne
done
clear
C)
E. Buchner
done
clear
D)
Northrop
done
clear
View Answer play_arrow
question_answer 126) Gambusia fish is :
A)
cat fish
done
clear
B)
sucker fish
done
clear
C)
mosquito fish
done
clear
D)
flat fish
done
clear
View Answer play_arrow
question_answer 127) Brain depends on blood for the supply of:
A)
oxygen and glucose
done
clear
B)
oxygen and electrolytes
done
clear
C)
oxygen and ATP
done
clear
D)
ATP and glucose
done
clear
View Answer play_arrow
question_answer 128) Plague is caused by :
A)
Diplococcus pneumoniae
done
clear
B)
Yersinia pestis
done
clear
C)
Corynebacterium diptheriae
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 129) In Musa, inflorescence is:
A)
capitulum
done
clear
B)
corymb
done
clear
C)
spadix
done
clear
D)
polychasial cyme
done
clear
View Answer play_arrow
question_answer 130) Sexual phase of Plasmodium life cycle occur in:
A)
man
done
clear
B)
mosquito
done
clear
C)
pig
done
clear
D)
goat
done
clear
View Answer play_arrow
question_answer 131) PCR was developed by :
A)
Karry Mullis (1983)
done
clear
B)
E. Southern (1980)
done
clear
C)
Francis Collins (1985)
done
clear
D)
James D. Watson (1953)
done
clear
View Answer play_arrow
question_answer 132) Ragi is:
A)
Eleusine coracana
done
clear
B)
Hordeum vulgare
done
clear
C)
Pennisetum typhoides
done
clear
D)
Triticum aestivum
done
clear
View Answer play_arrow
question_answer 133) International Rice Research Institute (IRRI) is located at:
A)
Hyderabad (India)
done
clear
B)
Manila (Philippines)
done
clear
C)
New York (USA)
done
clear
D)
Tokyo (Japan)
done
clear
View Answer play_arrow
question_answer 134) Which hormone is responsible for ovulation?
A)
FSH
done
clear
B)
LH
done
clear
C)
Testosterone
done
clear
D)
Oestrogen
done
clear
View Answer play_arrow
question_answer 135) Centromere is also called :
A)
chromomere
done
clear
B)
secondary constriction
done
clear
C)
primary constriction
done
clear
D)
chromocentre
done
clear
View Answer play_arrow
question_answer 136) VCRC stand for:
A)
Vaccine Creation Research Centre
done
clear
B)
Vector Control Research Centre
done
clear
C)
Venum Control Research Centre
done
clear
D)
Vital Chemical Release Centre
done
clear
View Answer play_arrow
question_answer 137) An aquatic fern used as bio-fertilizer is :
A)
Marsilea
done
clear
B)
Azolla
done
clear
C)
Pteridium
done
clear
D)
Salvinia
done
clear
View Answer play_arrow
question_answer 138) Which of the following protozoan, attacks gums in man?
A)
Entamoeba gingivalis
done
clear
B)
Trichomonas
done
clear
C)
Giardia
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 139) IPM (Integrated Pest Management) involves :
A)
tissue culture
done
clear
B)
biological control
done
clear
C)
bio-fertilizers
done
clear
D)
confusion technique
done
clear
View Answer play_arrow
question_answer 140) Philosophic Zoologique was written by :
A)
Lamarck
done
clear
B)
de Vries
done
clear
C)
Mendel
done
clear
D)
Spencer
done
clear
View Answer play_arrow
question_answer 141) Which of the following is correctly matched?
A)
Anopheles-Malaria
done
clear
B)
House fly-Yellow fever
done
clear
C)
Body loose-Typhoid
done
clear
D)
Sandfly-Plague
done
clear
View Answer play_arrow
question_answer 142) Incomplete breakdown of sugar in anaerobic respiration forms :
A)
glucose and \[C{{O}_{2}}\]
done
clear
B)
alcohol and \[C{{O}_{2}}\]
done
clear
C)
water and \[C{{O}_{2}}\]
done
clear
D)
fructose and water
done
clear
View Answer play_arrow
question_answer 143) Life cycle of Plasmodium is :
A)
monogenetic
done
clear
B)
digenetic
done
clear
C)
trigenetic
done
clear
D)
polygenetic
done
clear
View Answer play_arrow
question_answer 144) A child with mother of A blood group and father of AB blood group, will not have the following blood group :
A)
B
done
clear
B)
A
done
clear
C)
AB
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 145) What is correct about test tube baby?
A)
Fertilization outside and gestation inside womb of mother
done
clear
B)
Fertilization inside female genital tract and growth in test tube
done
clear
C)
Rearing of prematurely born baby in incubator
done
clear
D)
Both fertilization and -development are effected outside the female genital tract
done
clear
View Answer play_arrow
question_answer 146) Most important component of oral contraceptive is :
A)
LH
done
clear
B)
GH
done
clear
C)
thyroxine
done
clear
D)
progesterone
done
clear
View Answer play_arrow
question_answer 147) Purpose of tubectomy is to prevent:
A)
fertilization
done
clear
B)
coitus
done
clear
C)
egg formation
done
clear
D)
embryonic development
done
clear
View Answer play_arrow
question_answer 148) Fossil man, who made cave panitings is :
A)
Java man
done
clear
B)
Neanderthal man
done
clear
C)
Cro-magnon man
done
clear
D)
Peking man
done
clear
View Answer play_arrow
question_answer 149) Calvin cycle occurs in :
A)
cytoplasm
done
clear
B)
chloroplasts
done
clear
C)
mitochondria
done
clear
D)
glyoxysomes
done
clear
View Answer play_arrow
question_answer 150) Which of the following enzyme, is used to cut DNA strand at specific site?
A)
DNA ligase
done
clear
B)
DNA polymerase
done
clear
C)
RNA polymerase
done
clear
D)
Restriction endonuclease
done
clear
View Answer play_arrow
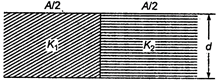 The capacitance of capacitor will be:
The capacitance of capacitor will be: