question_answer 1) A thin circular ring of mass M and radius R is rotating about its axis with a constant angular speed\[\omega \]. Two blocks, each of mass m are attached gently to the opposite ends of a diameter of the ring the angular speed of the ring will be :
A)
\[\frac{M+2\,m}{M}\omega \]
done
clear
B)
\[\frac{M}{M+2\,m}\omega \]
done
clear
C)
\[\frac{M-2\,m}{M+2\,m}\omega \]
done
clear
D)
\[\frac{2\,M}{M+2\,m}\omega \]
done
clear
View Answer play_arrow
question_answer 2) In a pressure cooker, the food is prepared quickly, the reason is that the :
A)
boiling point of water is raised due to increase in volume
done
clear
B)
boiling point of water is raised due to the increase in pressure
done
clear
C)
boiling point of water decreases due to increase in pressure
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 3) In the equation \[\left( p+\frac{a}{{{V}^{2}}} \right)\,(V-b)=RT\], where P = pressure, V = volume, a and b are constants, the dimensions of a are :
A)
\[[M{{L}^{5}}{{T}^{-1}}]\]
done
clear
B)
\[[M{{L}^{-5}}{{T}^{-1}}]\]
done
clear
C)
\[[M{{L}^{5}}{{T}^{-2}}]\]
done
clear
D)
\[[{{M}^{-1}}{{L}^{5}}{{T}^{2}}]\]
done
clear
View Answer play_arrow
question_answer 4) Two bulbs have wattage 250 W and 100 W respectively, each rated at 220 V. The ratio of resistances between the two bulbs will be :
A)
2 : 5
done
clear
B)
5 : 2
done
clear
C)
3 : 4
done
clear
D)
4 : 3
done
clear
View Answer play_arrow
question_answer 5) When a current in coil changes from 4 A to 2 A in 0.05 s, emf of 8 V is induced in the coil. The coefficient of self-inductance in the coil is :
A)
0.8 H
done
clear
B)
0.4 H
done
clear
C)
0.2 H
done
clear
D)
0.1H
done
clear
View Answer play_arrow
question_answer 6) If a sphere is rolling, the ratio of its rotational to total energy is given by:
A)
2 : 7
done
clear
B)
10 : 7
done
clear
C)
7 : 10
done
clear
D)
2 : 5
done
clear
View Answer play_arrow
question_answer 7) Water is flowing on the blades of a turbine at a rate of 100 kg/s from a certain spring. If the height of the spring be 100 m, the power transferred to the turbine will be :
A)
100 W
done
clear
B)
1 kW
done
clear
C)
10 kW
done
clear
D)
100 kW
done
clear
View Answer play_arrow
question_answer 8) A bullet \[({{m}_{i}}=25\,g)\] fired with a velocity 400 m/s gets embedded into a bag of sand \[({{m}_{2}}=4.9\,kg)\] suspended by a rope. The velocity gained by the bag is nearly :
A)
2 m/s
done
clear
B)
4 m/s
done
clear
C)
8 m/s
done
clear
D)
0.2 m/s
done
clear
View Answer play_arrow
question_answer 9) The work done to form a layer of soap solution of size \[10\times 10\,cm\] will be : (surface tension of soap solution is \[3\times {{10}^{-2}}N/m\])
A)
\[6\times {{10}^{-2}}J\]
done
clear
B)
\[6\times {{10}^{-4}}J\]
done
clear
C)
\[3\times {{10}^{-2}}J\]
done
clear
D)
\[3\times {{10}^{-4}}J\]
done
clear
View Answer play_arrow
question_answer 10) In a transistor \[\beta =100\]. The value of a is :
A)
1
done
clear
B)
0.99
done
clear
C)
0.1
done
clear
D)
0.01
done
clear
View Answer play_arrow
question_answer 11) A bomb of mass 9 kg at rest explodes into two pieces of mass 4 kg and 5 kg. The velocity of 4 kg mass is 15 m/s. What is the kinetic energy of 5 kg mass?
A)
Zero
done
clear
B)
562.5 J
done
clear
C)
360 J
done
clear
D)
180 J
done
clear
View Answer play_arrow
question_answer 12) The velocity of sound at \[{{0}^{o}}C\] is 332 m/s. At what temperature will it be 664 m/s?
A)
\[{{273}^{o}}C\]
done
clear
B)
\[{{546}^{o}}C\]
done
clear
C)
\[{{1092}^{o}}C\]
done
clear
D)
\[{{819}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 13) The equation of a wave moving on a string may be written as \[y=3\cos \pi (100\,t-x)\] where \[x,y\]are in cm and t in second. Its wavelength will be:
A)
5 cm
done
clear
B)
2 cm
done
clear
C)
100 cm
done
clear
D)
3 cm
done
clear
View Answer play_arrow
question_answer 14) A train moves towards a stationary observer with speed 34 m/s. The train sounds a whistle and its frequency registered by the observer is\[{{f}_{1}}\]. If the trains speed is reduced to 17 m/s the frequency registered is \[{{f}_{2}}\]. If the speed of sound is 340 m/s, the ratio \[{{f}_{1}}/{{f}_{2}}\] is :
A)
\[\frac{19}{18}\]
done
clear
B)
\[\frac{18}{19}\]
done
clear
C)
\[\frac{1}{2}\]
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 15) In a closed organ pipe which of the following notes is not present, if fundamental note is 50 Hz?
A)
100 Hz
done
clear
B)
150 Hz
done
clear
C)
250 Hz
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 16) A monoatomic gas \[(\gamma =5/3)\] at pressure P is suddenly compressed to \[\frac{1}{8}\]th of its volume adiabatically, the pressure of gas is :
A)
\[\frac{43}{3}P\]
done
clear
B)
8 P
done
clear
C)
32 P
done
clear
D)
\[\frac{24}{5}P\]
done
clear
View Answer play_arrow
question_answer 17) An ideal Carnot engine whose efficiency is 40% receives heat at 500 K. If the efficiency is to be 50% the intake temperature for this same exhaust temperature is :
A)
600 K
done
clear
B)
800 K
done
clear
C)
900 K
done
clear
D)
1000 K
done
clear
View Answer play_arrow
question_answer 18) A mixture of gas contains 8 g of oxygen and 7 g of nitrogen. To use the equation \[PV=nRT\], the value of n of such mixture will be :
A)
\[\frac{1}{2}\]
done
clear
B)
\[\frac{1}{4}\]
done
clear
C)
2
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 19) How many times diatomic gas is should be expanded adiabatically, so as to reduce the root mean square velocity to half?
A)
64
done
clear
B)
32
done
clear
C)
16
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 20) The masses of three wires of copper are in the ratio 1:2:3 and their lengths are in the ratio 3 : 2 : 1. The ratio of their resistances is :
A)
3 : 2 : 1
done
clear
B)
1 : 2 : 3
done
clear
C)
27 : 6 : 1
done
clear
D)
1 : 6 : 27
done
clear
View Answer play_arrow
question_answer 21) If the length of a conductor is halved, then the conductance will be :
A)
quadrupled
done
clear
B)
doubled
done
clear
C)
halved
done
clear
D)
unchanged
done
clear
View Answer play_arrow
question_answer 22) The vertical component of earths magnetic field is zero at a place whose angle of dip is :
A)
\[{{90}^{o}}\]
done
clear
B)
\[{{60}^{o}}\]
done
clear
C)
\[{{45}^{o}}\]
done
clear
D)
\[{{0}^{o}}\]
done
clear
View Answer play_arrow
question_answer 23) Energy stored in a coil of self-inductance 40 mH carrying a steady current of 2 A is :
A)
80 J
done
clear
B)
0.08 J
done
clear
C)
0.8 J
done
clear
D)
8 J
done
clear
View Answer play_arrow
question_answer 24) Resistance of rod is \[1\,\,\Omega \]. It is bent in the form of square. What is the resistance across adjoint corners?
A)
\[1\,\,\Omega \]
done
clear
B)
\[3\,\,\Omega \]
done
clear
C)
\[\frac{3}{16}\,\,\Omega \]
done
clear
D)
\[\frac{3}{4}\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 25)
A capacitor of capacitance \[1\,\mu F\] is filled with two dielectrics of dielectric constants 4 and 6. What is the new capacitance?
A)
\[10\,\mu F\]
done
clear
B)
\[5\,\mu F\]
done
clear
C)
\[4\,\mu F\]
done
clear
D)
\[7\,\mu F\]
done
clear
View Answer play_arrow
question_answer 26) The focal length of lens is 5 cm. If the least distance of distinct vision is 25 cm the magnification is :
A)
2.1
done
clear
B)
1
done
clear
C)
1.1
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 27) The critical angle for a medium is \[{{45}^{o}}\]. The refractive index will be :
A)
\[\frac{2}{\sqrt{3}}\]
done
clear
B)
\[\frac{\sqrt{3}}{2}\]
done
clear
C)
\[\sqrt{2}\]
done
clear
D)
\[\frac{1}{\sqrt{2}}\]-
done
clear
View Answer play_arrow
question_answer 28) The specific resistance of wire of length 1 m, area of cross-section 0.5 \[{{m}^{2}}\] is \[25\,\,\mu \,\Omega \], The resistance of the wire will be :
A)
\[5\times {{10}^{-5}}\Omega \]
done
clear
B)
\[3\times {{10}^{6}}\Omega \]
done
clear
C)
\[46\times {{10}^{6}}\Omega \]
done
clear
D)
\[2\times {{10}^{6}}\Omega \]
done
clear
View Answer play_arrow
question_answer 29) Two identical wires A and B have the same length L and carry the same current \[I\]. Wire A is bent into a circle of radius R and wire B is bent to form a square of side a. If \[{{B}_{1}}\] and \[{{B}_{2}}\]are the values of magnetic induction at the centre of the circle and the centre of the square respectively, then the ratio of\[{{B}_{1}}/{{B}_{2}}\]is :
A)
\[\frac{{{\pi }^{2}}}{8}\]
done
clear
B)
\[\frac{{{\pi }^{2}}}{8\sqrt{2}}\]
done
clear
C)
\[\frac{{{\pi }^{2}}}{16}\]
done
clear
D)
\[\frac{{{\pi }^{2}}}{16\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 30) The force acting on a pole of pole strength 1 Am is \[{{10}^{-4}}N\]. The magnetic field at that point is :
A)
\[11\times {{10}^{-4}}T\]
done
clear
B)
\[10\times {{10}^{-4}}T\]
done
clear
C)
\[0.1\times {{10}^{-4}}T\]
done
clear
D)
\[1\times {{10}^{-4}}T\]
done
clear
View Answer play_arrow
question_answer 31) A tuning fork produces 5 beats/s with another tuning fork B of frequency 256 Hz. On filing fork A only 2 beats are heard per second, the frequency of A before filing is :
A)
251 Hz
done
clear
B)
254 Hz
done
clear
C)
261 Hz
done
clear
D)
256 Hz
done
clear
View Answer play_arrow
question_answer 32) Number of photons of wavelength 600 nm emitted per second by an electric bulb of power 50 W is : (Take \[h=6.6\times {{10}^{-34}}J-s\])
A)
\[{{10}^{19}}\]
done
clear
B)
\[2\times {{10}^{20}}\]
done
clear
C)
60
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 33) Half-life of radioactive substance is 3.20 h. What is the time taken for a 75% of substance to be used?
A)
6.38 h
done
clear
B)
12 h
done
clear
C)
4.18 days
done
clear
D)
1.2 days
done
clear
View Answer play_arrow
question_answer 34) TV waves have wavelength range of 1-10 m. Their frequency range will be :
A)
3-30 MHz
done
clear
B)
30-300 MHz
done
clear
C)
3-3000 MHz
done
clear
D)
300-3000 MHz
done
clear
View Answer play_arrow
question_answer 35) Reactance of a capacitor of capacitance C \[\mu F\]for AC frequency \[\frac{400}{\pi }\] Hz is 25 D. The value of C is:
A)
\[50\,\mu F\]
done
clear
B)
\[25\,\mu F\]
done
clear
C)
\[100\,\mu F\]
done
clear
D)
\[75\,\mu F\]
done
clear
View Answer play_arrow
question_answer 36) An electron and a proton have same de-Broglie wavelength, then kinetic energy of the electron is :
A)
infinite
done
clear
B)
equal to KE of proton
done
clear
C)
zero
done
clear
D)
greater than KE of proton
done
clear
View Answer play_arrow
question_answer 37) Relative permeability of iron is 5500. Its magnetic susceptibility is :
A)
5501
done
clear
B)
\[5500\times {{10}^{-7}}\]
done
clear
C)
\[5500\times {{10}^{7}}\]
done
clear
D)
5499
done
clear
View Answer play_arrow
question_answer 38) An example for diamagnetic substances is :
A)
gold
done
clear
B)
nickel
done
clear
C)
aluminium
done
clear
D)
iron
done
clear
View Answer play_arrow
question_answer 39) The impurity atom that should be added to germanium to make it n-type semiconductor is:
A)
aluminium
done
clear
B)
phosphorus
done
clear
C)
boron
done
clear
D)
indium
done
clear
View Answer play_arrow
question_answer 40) An atom of mass number A and atomic number Z emits successively an \[\alpha \]-particle and a, \[\beta \]-particle and y-rays. The mass number and atomic number of the end product, will be :
A)
A - 4, Z - 1
done
clear
B)
A - 4, Z - 2
done
clear
C)
A - 1, Z - 4
done
clear
D)
A, Z + 1
done
clear
View Answer play_arrow
question_answer 41) When R is Rydbergs constant for hydrogen, the wave number of the first line in the Lyman series is :
A)
\[\frac{R}{4}\]
done
clear
B)
\[\frac{3R}{4}\]
done
clear
C)
\[\frac{R}{2}\]
done
clear
D)
2R
done
clear
View Answer play_arrow
question_answer 42) A source of sound of frequency 256 Hz is moving towards a wall with a velocity of 5 m/s. The velocity of sound is 330 m/s. The number of beats heard by an observer moving along with the sounding object is :
A)
\[256\times \frac{330}{325}-256\]
done
clear
B)
\[256-\frac{256\times 330}{335}\]
done
clear
C)
\[\frac{256\times 330}{325}-\frac{256\times 330}{325}\]
done
clear
D)
\[256\times \frac{335}{325}-256\]
done
clear
View Answer play_arrow
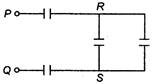
question_answer 43)
The effective capacitance between the points P and Q in the circuit shown in figure, is (capacitance of each capacitor is 1 \[\mu F\]) :
A)
0.4 \[\mu F\]
done
clear
B)
3 \[\mu F\]
done
clear
C)
4 \[\mu F\]
done
clear
D)
2 \[\mu F\]
done
clear
View Answer play_arrow
question_answer 44) What is the resistance of a 40 W lamp which is lighted at full brilliance by a current of \[\frac{1}{3}A\]?
A)
\[450\,\,\Omega \]
done
clear
B)
\[360\,\,\Omega \]
done
clear
C)
\[120\,\,\Omega \]
done
clear
D)
\[13.33\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 45) The energy gap of silicon is 1.14 eV. The maximum wavelength at which silicon will begin absorbing energy is :
A)
18.855 \[\overset{o}{\mathop{A}}\,\]
done
clear
B)
108.55 \[\overset{o}{\mathop{A}}\,\]
done
clear
C)
1085.5 \[\overset{o}{\mathop{A}}\,\]
done
clear
D)
10855 \[\overset{o}{\mathop{A}}\,\]
done
clear
View Answer play_arrow
question_answer 46)
Assuming that the junction diode is ideal, the current through the diode is :
A)
0.02 A
done
clear
B)
2 A
done
clear
C)
0.2 A
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 47) With a resistance R connected in series with galvanometer of resistance \[100\,\,\Omega \], it acts as a voltmeter of range 0 - V. To double the range a resistance of \[1000\,\,\Omega \] is to be connected in series with R. Then the value of-R is:
A)
\[400\,\,\Omega \]
done
clear
B)
\[900\,\,\Omega \]
done
clear
C)
\[1100\,\,\Omega \]
done
clear
D)
\[1000\,\,\Omega \]
done
clear
View Answer play_arrow
question_answer 48) An a-particle can be represented by a :
A)
hydrogen atom
done
clear
B)
helium ion
done
clear
C)
hydrogen ion
done
clear
D)
helium nucleus
done
clear
View Answer play_arrow
question_answer 49) Coefficient of coupling between two coils of self-inductances \[{{L}_{1}}\] and \[{{L}_{2}}\] is unity. It means:
A)
50% flux of \[{{L}_{1}}\] is linked with \[{{L}_{2}}\]
done
clear
B)
100% flux of \[{{L}_{1}}\] is linked with \[{{L}_{2}}\]
done
clear
C)
\[\sqrt{{{L}_{1}}}\] time of flux of \[{{L}_{1}}\] is linked with \[{{L}_{2}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 50) State which of the following is correct?
A)
\[{{g}_{m}}=\mu \times {{r}_{p}}\]
done
clear
B)
\[{{r}_{p}}=\mu \times {{g}_{m}}\]
done
clear
C)
\[\mu ={{r}_{p}}\times {{g}_{m}}\]
done
clear
D)
\[\mu =\frac{{{r}_{p}}}{{{g}_{m}}}\]
done
clear
View Answer play_arrow
question_answer 51) Modern periodic table is based on atomic number of the elements. The significance of atomic number was proved by the experiment of:
A)
discovery of X-ray by Roentgen
done
clear
B)
Mullikans oil drop experiment
done
clear
C)
Braggs work on X-ray diffraction
done
clear
D)
Mosleys work on X-ray spectra
done
clear
View Answer play_arrow
question_answer 52) The shape of \[Xe{{O}_{4}}\] molecule is tetrahedral due to:
A)
sp
done
clear
B)
\[s{{p}^{2}}\]
done
clear
C)
\[s{{p}^{3}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 53) The monomer of natural rubber is :
A)
isoprene
done
clear
B)
adipic acid
done
clear
C)
vinyl chloride
done
clear
D)
acrylonitrile
done
clear
View Answer play_arrow
question_answer 54) Iodoform test is given by:
A)
1-propanol
done
clear
B)
methanol
done
clear
C)
2-butanol
done
clear
D)
1-butanol
done
clear
View Answer play_arrow
question_answer 55) \[0.3\,M\,{{H}_{3}}P{{O}_{3}}\] shows the normality of:
A)
0.3
done
clear
B)
0.6
done
clear
C)
0.9
done
clear
D)
0.1
done
clear
View Answer play_arrow
question_answer 56) At 300 K, the ratio of the average molecular kinetic energy of \[U{{F}_{6}}\] to that of \[{{H}_{2}}\] is :
A)
2 : 7
done
clear
B)
7 : 2
done
clear
C)
6 : 2
done
clear
D)
1 : 1
done
clear
View Answer play_arrow
question_answer 57) Graphite and diamond are :
A)
polymorphs
done
clear
B)
allotropes
done
clear
C)
different crystal structure
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 58) The correct alternate for the cells spontaneous reaction is :
A)
\[E_{cell}^{o}\] cell should be infinite
done
clear
B)
\[E_{cell}^{o}\] cell should be zero
done
clear
C)
\[E_{cell}^{o}\] cell should be positive
done
clear
D)
\[E_{cell}^{o}\] cell should be negative
done
clear
View Answer play_arrow
question_answer 59) The maximum amount of sulphur is given by :
A)
\[AgS\,({{K}_{sp}}=6\times {{10}^{-29}})\]
done
clear
B)
\[CuS\,({{K}_{sp}}=4\times {{10}^{-27}})\]
done
clear
C)
\[PbS\,({{K}_{sp}}=8\times {{10}^{-28}})\]
done
clear
D)
\[ZnS\,({{K}_{sp}}=8\times {{10}^{-30}})\]
done
clear
View Answer play_arrow
question_answer 60) The highest electronegative element is :
A)
carbon
done
clear
B)
oxygen
done
clear
C)
fluorine
done
clear
D)
nitrogen
done
clear
View Answer play_arrow
question_answer 61) Bromine can be liberated from KBr solution by the action of:
A)
chlorine
done
clear
B)
NaCI
done
clear
C)
KI
done
clear
D)
iodine solution
done
clear
View Answer play_arrow
question_answer 62) The aldehyde can be detected by which of the following?
A)
Tollens reagent
done
clear
B)
Fehlings solution
done
clear
C)
Schiffs reagent
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 63) Froth floatation method is used for the concentration of:
A)
sulphide ore
done
clear
B)
oxide ore
done
clear
C)
chloride ore
done
clear
D)
amalgams
done
clear
View Answer play_arrow
question_answer 64) The zero dipole moment is shown by :
A)
cis-1, 2-dichloroethylene
done
clear
B)
trans-1, 2- dichloroethylene
done
clear
C)
1, 1-dichloroethylene
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 65) The correct order of electron affinities of S, N, 0 and \[Cl\] is :
A)
\[O=Cl<S=N\]
done
clear
B)
\[O<S<Cl<N\]
done
clear
C)
\[N<O<S<Cl\]
done
clear
D)
\[O<N<Cl<S\]
done
clear
View Answer play_arrow
question_answer 66) In isotones the atomic number and mass numbers are :
A)
same
done
clear
B)
different
done
clear
C)
equal
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 67) One mole of \[C{{O}_{2}}\] contains :
A)
\[18.1\times {{10}^{23}}\] molecules of \[C{{O}_{2}}\]
done
clear
B)
\[6.02\times {{10}^{23}}\] atom of carbon
done
clear
C)
3 g of carbon
done
clear
D)
\[6.02\times {{10}^{23}}\] atoms of oxygen
done
clear
View Answer play_arrow
question_answer 68) The interhalogen compound not formed is :
A)
\[ICl\]
done
clear
B)
\[I{{F}_{5}}\]
done
clear
C)
\[Br{{F}_{5}}\]
done
clear
D)
\[BrC{{l}_{7}}\]
done
clear
View Answer play_arrow
question_answer 69) The element prepared by electrolysis is :
A)
sodium
done
clear
B)
chlorine
done
clear
C)
fluorine
done
clear
D)
bromine
done
clear
View Answer play_arrow
question_answer 70) The element chlorine consists of a mixture of \[75.53%{{\,}_{17}}C{{l}^{35}}\] and \[24.47%{{\,}_{17}}C{{l}^{37}}\] having a mass of 34.97 amu and 36.95 amu, respectively. The atomic weight of chlorine is:
A)
35.25
done
clear
B)
35.45
done
clear
C)
36.25
done
clear
D)
36.45
done
clear
View Answer play_arrow
question_answer 71) The solubility of \[A{{g}_{2}}S\] is \[[{{K}_{sp}}=256\times {{10}^{-6}}]\] :
A)
\[4\times {{10}^{-2}}\]
done
clear
B)
\[4\times {{10}^{-3}}\]
done
clear
C)
\[0.4\times {{10}^{-2}}\]
done
clear
D)
\[0.4\times {{10}^{-3}}\]
done
clear
View Answer play_arrow
question_answer 72) Marsh gas contains :
A)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
D)
\[C{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 73) The ion present in Nesslers reagent is :
A)
\[Hg{{I}_{2}}\]
done
clear
B)
\[HgI_{4}^{2-}\]
done
clear
C)
\[H{{g}^{2+}}\]
done
clear
D)
\[H{{g}^{+}}\]
done
clear
View Answer play_arrow
question_answer 74) Vitamin \[{{B}_{12}}\] contains :
A)
Cu
done
clear
B)
Fe
done
clear
C)
Mg
done
clear
D)
Co
done
clear
View Answer play_arrow
question_answer 75) 720 g of water contains how many moles of water?
A)
20
done
clear
B)
40
done
clear
C)
60
done
clear
D)
80
done
clear
View Answer play_arrow
question_answer 76) \[Zn(s)+C{{u}^{2+}}(aq)\xrightarrow{{}}Cu(s)+Z{{n}^{2+}}(aq)\] The emf of above reaction is 1.10 V at \[{{25}^{o}}C\]. Find emf of cell well when \[[Z{{n}^{2+}}]=0.1\,M\] \[[C{{u}^{2+}}]=0.1M\]
A)
1.10 V
done
clear
B)
-1.10 V
done
clear
C)
0.110 V
done
clear
D)
-0.110 V
done
clear
View Answer play_arrow
question_answer 77) The colour of iodine solution is discharged by shaking it with aqueous solution of:
A)
sodium sulphite
done
clear
B)
sulphuric acid
done
clear
C)
sodium sulphate
done
clear
D)
sodium thiosulphite
done
clear
View Answer play_arrow
question_answer 78) Nitrobenzene on treatment with \[B{{r}_{2}}\]produce :
A)
o, p-bromobenzene
done
clear
B)
p-b-bromonitrobenzene
done
clear
C)
m-bromonitrobenzene
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 79) The entropy change \[(\Delta S)\] in a system increases as :
A)
solid < liquid < gas
done
clear
B)
liquid < gas < solid
done
clear
C)
solid < gas < liquid
done
clear
D)
gas < solid < liquid
done
clear
View Answer play_arrow
question_answer 80) The element having maximum hydration energy is :
A)
Na
done
clear
B)
K
done
clear
C)
Rb
done
clear
D)
Cs
done
clear
View Answer play_arrow
question_answer 81) The total number of electrons present in all the p-orbitals of bromine is :
A)
7
done
clear
B)
17
done
clear
C)
27
done
clear
D)
37
done
clear
View Answer play_arrow
question_answer 82) The rate constant of third order reaction is :
A)
\[mol\,{{L}^{-1}}{{s}^{-1}}\]
done
clear
B)
\[mo{{l}^{-2}}\,{{L}^{2}}{{T}^{-1}}\]
done
clear
C)
\[mo{{l}^{-1}}\,L\,{{s}^{-1}}\]
done
clear
D)
\[mo{{l}^{-2}}\,{{L}^{-1}}\,{{s}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 83) By the reduction of carbonyl compounds the alcohol not obtained is :
A)
primary alcohol
done
clear
B)
secondary alcohol
done
clear
C)
tertiary alcohol
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 84) The mixture of gases used for asthma patients is :
A)
\[{{O}_{2}}+He\]
done
clear
B)
\[{{O}_{2}}+Ne\]
done
clear
C)
\[{{O}_{2}}+{{H}_{2}}\]
done
clear
D)
\[{{O}_{2}}+Ar\]
done
clear
View Answer play_arrow
question_answer 85) Acetate ion have :
A)
two carbon, oxygen double bond
done
clear
B)
two carbon, oxygen single bond
done
clear
C)
one carbon, oxygen single bond and one carbon, oxygen double bond
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 86) The difference between energy of product and energy of reactant is called :
A)
entropy
done
clear
B)
heat content
done
clear
C)
heat of fusion
done
clear
D)
activation energy
done
clear
View Answer play_arrow
question_answer 87) The most covalent bond is :
A)
C-S
done
clear
B)
C-0
done
clear
C)
C-F
done
clear
D)
C-Br
done
clear
View Answer play_arrow
question_answer 88) A solution is made up of 10 mL of 0.1 N NaOH and 10 mL of \[0.05\,N\,{{H}_{2}}S{{O}_{4}}\]. The pH of solution is :
A)
7
done
clear
B)
> 7
done
clear
C)
< 7
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 89) The addition of 1% alcohol to chloroform acts as a :
A)
positive catalyst
done
clear
B)
negative catalyst
done
clear
C)
autocatalyst
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 90) \[C{{H}_{3}}-CH=CH-C{{H}_{3}}\] on ozonolysis produce:
A)
acetaldehyde
done
clear
B)
mixture of acetaldehyde and ethyl alcohol
done
clear
C)
ethyl alcohol
done
clear
D)
mixture of acetone and acetaldehyde
done
clear
View Answer play_arrow
question_answer 91) Nitroalkane which do not exhibit tautomerism is :
A)
\[{{3}^{o}}-\]nitroalkane
done
clear
B)
\[{{2}^{o}}-\]nitroalkane
done
clear
C)
\[{{1}^{o}}-\]nitroalkane
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 92) The paramagnetic element is :
A)
\[N{{i}^{2+}}\]
done
clear
B)
\[S{{c}^{3+}}\]
done
clear
C)
\[C{{u}^{+}}\]
done
clear
D)
\[Z{{n}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 93) Addition of HBr on propene produce :
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Br\]
done
clear
B)
\[C{{H}_{2}}BrC{{H}_{2}}C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{3}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 94) Which of the following, is not a planar compound?
A)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
B)
\[N{{H}_{3}}\]
done
clear
C)
\[{{N}_{2}}O\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 95) The saturated pentane have total number of isomers are :
A)
5
done
clear
B)
4
done
clear
C)
6
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 96) \[Mn{{O}_{2}}(s)+4HCl\,(aq)\xrightarrow{Heat}\] \[MnC{{l}_{2}}(aq)+2{{H}_{2}}O+C{{l}_{2}}(g)\] The equivalent weight of \[Mn{{O}_{2}}\] in the above reaction is :
A)
23.4
done
clear
B)
33.6
done
clear
C)
43.5
done
clear
D)
53.6
done
clear
View Answer play_arrow
question_answer 97) Tetraethyl lead is used as :
A)
fire extinguisher
done
clear
B)
mosquito repellent
done
clear
C)
petroleum additive
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 98) 4 moles of A are mixed with 4 moles of B and 2 moles of C are formed at equilibrium according to following reaction, \[A+BC+D\]. The value of equilibrium constant is :
A)
1
done
clear
B)
4
done
clear
C)
\[\frac{1}{4}\]
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 99) The vapour pressure of benzene at a certain temperature is 200 mm Hg. At the same temperature the vapour pressure of a solution containing 2 g of non volatile non electrolyte solid in 78 g of benzene is 195 mm Hg. The molecular weight of solid is :
A)
20
done
clear
B)
40
done
clear
C)
60
done
clear
D)
80
done
clear
View Answer play_arrow
question_answer 100) The metal not present in hard water is :
A)
Ca
done
clear
B)
Na
done
clear
C)
Mg
done
clear
D)
Fe
done
clear
View Answer play_arrow
question_answer 101) Cohesion-tension theory regarding ascent of sap was given by :
A)
Dixon and Jolly
done
clear
B)
J.C. Bose
done
clear
C)
Christian Wolf
done
clear
D)
Godlewski
done
clear
View Answer play_arrow
question_answer 102) Which among the following is a synthetic plant hormone?
A)
IAA
done
clear
B)
GA
done
clear
C)
2, 4-D
done
clear
D)
ABA
done
clear
View Answer play_arrow
question_answer 103) Waggle dance of honey bee indicates direction of:
A)
food source
done
clear
B)
mating call
done
clear
C)
enemy
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 104) What is the benefit from pollen culture?
A)
Production of hybrid species
done
clear
B)
Rare plant species can be preserve
done
clear
C)
Haploid plants can be produced
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 105) Embryo sac of an angiospermic plant is:
A)
male gametophyte
done
clear
B)
female gametophyte
done
clear
C)
male sporophyte
done
clear
D)
female sporophyte
done
clear
View Answer play_arrow
question_answer 106) \[S{{O}_{2}}\] affects :
A)
nuclear membrane
done
clear
B)
cell wall
done
clear
C)
mitochondria
done
clear
D)
all membrane system
done
clear
View Answer play_arrow
question_answer 107) Which among the following is an air pollution indicator?
A)
Algae
done
clear
B)
Lichen
done
clear
C)
Fungi
done
clear
D)
Ferns
done
clear
View Answer play_arrow
question_answer 108) Biological equilibrium can be maintained between :
A)
producer and consumer
done
clear
B)
consumer and decomposer
done
clear
C)
producer and decomposer
done
clear
D)
producer, consumer and decomposer
done
clear
View Answer play_arrow
question_answer 109) Velamen tissue is found in :
A)
mesophytes
done
clear
B)
epiphytes
done
clear
C)
hydrophytes
done
clear
D)
xerophytes-
done
clear
View Answer play_arrow
question_answer 110) Pneumatophores are characteristic feature of:
A)
Hydrilla
done
clear
B)
Rhizophora
done
clear
C)
Typha
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 111) Which enzyme is proved to be very useful in genetic engineering?
A)
Telomerases
done
clear
B)
Polymerases
done
clear
C)
Endonucleases
done
clear
D)
Hydrolases
done
clear
View Answer play_arrow
question_answer 112) Phytoplanktons are an important biotic component of:
A)
grassland ecosystem
done
clear
B)
pond ecosystem
done
clear
C)
forest ecosystem
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 113) Which one is produced during cyclic photo-phosphorylation ?
A)
ATP and \[NADP{{H}_{2}}\]
done
clear
B)
ATP only
done
clear
C)
ATP and \[{{O}_{2}}\]
done
clear
D)
\[NADP{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 114) Glycolysis takes place in ;
A)
cytoplasm
done
clear
B)
nucleus
done
clear
C)
plastid
done
clear
D)
mitochondria
done
clear
View Answer play_arrow
question_answer 115) Which one is hexose sugar?
A)
Mannose
done
clear
B)
Galactose
done
clear
C)
Both [a] and [ b]
done
clear
D)
Cellulose
done
clear
View Answer play_arrow
question_answer 116) Tetradynamous conditions is found in family :
A)
Malvaceae
done
clear
B)
Solanaceae
done
clear
C)
Brassicaceae
done
clear
D)
Liliaceae
done
clear
View Answer play_arrow
question_answer 117) Nucleoside is :
A)
polymer of nucleic acid
done
clear
B)
phosphoric acid + ba2se
done
clear
C)
phosphoric acid + sugar + base
done
clear
D)
sugar + base
done
clear
View Answer play_arrow
question_answer 118) In a fully turgid plant cell which one is zero?
A)
Equal to turgor pressure
done
clear
B)
Equal to wall pressure
done
clear
C)
DPD
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 119) Coconut is which type of fruit?
A)
Drupe
done
clear
B)
Cypsela
done
clear
C)
Berry
done
clear
D)
Cremocarp
done
clear
View Answer play_arrow
question_answer 120) Formation of sex cell was first seen in :
A)
pteridophytes
done
clear
B)
bryophytes
done
clear
C)
gymnosperm
done
clear
D)
angiosperm
done
clear
View Answer play_arrow
question_answer 121) DNA is a genetic material was proved by
A)
Griffith
done
clear
B)
Linus Pauling
done
clear
C)
Meselson and Stahl
done
clear
D)
Watson
done
clear
View Answer play_arrow
question_answer 122) Jumping genes are referred to :
A)
exon
done
clear
B)
intron
done
clear
C)
cytoplasmic particles
done
clear
D)
transposable
done
clear
View Answer play_arrow
question_answer 123) Which organelle is not found in an animal cell?
A)
Peroxysome
done
clear
B)
Ribosome
done
clear
C)
Lysosome
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 124) Haemophilia (sickle cell anaemia) is a:
A)
deficiency disorder
done
clear
B)
male sex disorder
done
clear
C)
female sex disorder
done
clear
D)
autosomal sex disorder
done
clear
View Answer play_arrow
question_answer 125) Kinetochore is :
A)
a type of chromophore
done
clear
B)
part of chromosome at centromere
done
clear
C)
part of centrosome
done
clear
D)
constituent of chromomere
done
clear
View Answer play_arrow
question_answer 126) Fully mature larva of frog respire by:
A)
skin
done
clear
B)
gills
done
clear
C)
lungs
done
clear
D)
buccal cavity
done
clear
View Answer play_arrow
question_answer 127) Haematocrit value gives :
A)
amount of RBC in blood
done
clear
B)
number of WBC in blood
done
clear
C)
amount of plasma in blood
done
clear
D)
haemoglobin concentration in blood
done
clear
View Answer play_arrow
question_answer 128) Different colours of frog skins are controlled by:
A)
nervous system
done
clear
B)
hormones
done
clear
C)
both [a] and [b]
done
clear
D)
different kinds of melanocytes
done
clear
View Answer play_arrow
question_answer 129) Sex determination ratio in an organism is given by \[\frac{X}{A}=15\], then organism will be:
A)
male
done
clear
B)
female
done
clear
C)
super female
done
clear
D)
intersex
done
clear
View Answer play_arrow
question_answer 130) Nissls granules are :
A)
RNA bodies
done
clear
B)
DNA
done
clear
C)
carbohydrate
done
clear
D)
protein
done
clear
View Answer play_arrow
question_answer 131) Tubulin fibre is present in :
A)
cilia
done
clear
B)
flagella
done
clear
C)
microtubuies
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 132) Rhabditiform is the larva of :
A)
Hydra
done
clear
B)
Platyhelminthes
done
clear
C)
Ascaris
done
clear
D)
Earthworm
done
clear
View Answer play_arrow
question_answer 133) Which among the following is not skeleton eye muscle?
A)
Medial reetus
done
clear
B)
Lateral reetus
done
clear
C)
Ciliary muscle
done
clear
D)
Oblique muscle
done
clear
View Answer play_arrow
question_answer 134) Soil which is carried by air and deposited :
A)
alluvial
done
clear
B)
colluvial
done
clear
C)
eolian
done
clear
D)
glacial
done
clear
View Answer play_arrow
question_answer 135) Kidney of frog is :
A)
pronephric
done
clear
B)
mesonephric
done
clear
C)
metanephric
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 136) Gonads in Hydra develops from :
A)
epithelio muscular cell
done
clear
B)
interstitial cells
done
clear
C)
archeocyte cells
done
clear
D)
singing cells
done
clear
View Answer play_arrow
question_answer 137) Sandfly spreads a particular type of disease by its :
A)
proboscis
done
clear
B)
salivary gland
done
clear
C)
mandible
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 138) Oak silkwrom is:
A)
Antherea roylei
done
clear
B)
Apis florea
done
clear
C)
Bombyx mori
done
clear
D)
Clarias batrachus
done
clear
View Answer play_arrow
question_answer 139) Which one causes water bloom?
A)
Red algae
done
clear
B)
Fungi
done
clear
C)
Green algae
done
clear
D)
Fern
done
clear
View Answer play_arrow
question_answer 140) Enterogastrone is present in :
A)
stomach
done
clear
B)
small intestine
done
clear
C)
oesophagus
done
clear
D)
both [a] and [b]
done
clear
View Answer play_arrow
question_answer 141) Gambusia feeds upon :
A)
maggots
done
clear
B)
insects
done
clear
C)
Hydra
done
clear
D)
larvae of mosquito
done
clear
View Answer play_arrow
question_answer 142) At which research institute medicine regarding antivenom is made ?
A)
CDRI (Lucknow)
done
clear
B)
AIIMS (Delhi)
done
clear
C)
HRT(Mumbai)
done
clear
D)
ITRC (Lucknow)
done
clear
View Answer play_arrow
question_answer 143) Sewage water can be purified for recycling with the action of:
A)
aquatic plant
done
clear
B)
penicillin
done
clear
C)
micro-organisms
done
clear
D)
fishes
done
clear
View Answer play_arrow
question_answer 144) Golden Sela Rice is a rice variety enriched in:
A)
P-carotene
done
clear
B)
lysine
done
clear
C)
vitamin- C
done
clear
D)
iron
done
clear
View Answer play_arrow
question_answer 145) Which of the following is true moss?
A)
Irish moss
done
clear
B)
Bog moss
done
clear
C)
Club moss
done
clear
D)
Reindeers moss
done
clear
View Answer play_arrow
question_answer 146) Factors relating to form and behaviour of the earths surface are called :
A)
edaphic
done
clear
B)
tophographic
done
clear
C)
climatic
done
clear
D)
biotic
done
clear
View Answer play_arrow
question_answer 147) Which ecological pyramid can never occur in an inverted form?
A)
Pyramid of number
done
clear
B)
Pyramid of biomass
done
clear
C)
Pyramid of energy
done
clear
D)
Pyramid species richness
done
clear
View Answer play_arrow
question_answer 148) Ophiasaurus belong to :
A)
Amphibia
done
clear
B)
Pisces
done
clear
C)
Reptilia
done
clear
D)
Mammalia
done
clear
View Answer play_arrow
question_answer 149) The poisnous fluid present in the nematocyst of Hydra is :
A)
toxin
done
clear
B)
venom
done
clear
C)
haemation
done
clear
D)
hypnotoxin
done
clear
View Answer play_arrow
question_answer 150) Epistasis implies :
A)
one pair of genes can completely mask the expression of another pair of genes
done
clear
B)
on pair of genes independently controls a particular phenotype
done
clear
C)
one parr of genes enhances the phenotype expression of another pair of genes
done
clear
D)
many genes collectively control a particular phenotype
done
clear
View Answer play_arrow