question_answer 1) The moving fan has a constant angular acceleration of\[\pi /6\]\[rad/{{s}^{2}}\]. If it starts from rest, the number of revolution it makes in one minute is
A)
1600
done
clear
B)
500
done
clear
C)
350
done
clear
D)
150
done
clear
View Answer play_arrow
question_answer 2) A ball of mass 200 gm, moving with a speed of 40 m/s, is deflected exactly with the same speed but at\[90{}^\circ \]with its incident direction after striking with a bat. If the striking time is 2 s, the average force acts on the ball is
A)
4.0 N
done
clear
B)
\[4/\sqrt{2}N\]
done
clear
C)
\[4\sqrt{2}N\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 3) A particle of mass m is moving in a circular path of constant radius r such that its centripetal acceleration\[{{a}_{c}}\]is varying with time \[t\] as\[{{a}_{c}}={{k}^{4}}r{{t}^{2}}\]where k is constant. The power delivered to the particle by the forces acting on it is
A)
\[m{{k}^{2}}{{r}^{2}}{{t}^{2}}\]
done
clear
B)
\[m{{k}^{2}}{{r}^{2}}t\]
done
clear
C)
\[\pi m{{k}^{2}}{{r}^{2}}{{t}^{2}}\]
done
clear
D)
\[m{{k}^{4}}{{r}^{2}}t\]
done
clear
View Answer play_arrow
question_answer 4) A projectile is thrown obliquely into the air from origin of a reference frame with y-axis vertically upward and\[x-\]axis perpendicular to y then equation of motion has the form
A)
\[y=ax+b{{x}^{2}}\]
done
clear
B)
\[y=a{{x}^{2}}+b{{x}^{3}}\]
done
clear
C)
\[y=a+bx\]
done
clear
D)
\[y=(a+b)x\]
done
clear
View Answer play_arrow
question_answer 5) The moment of inertia, of an annular cylinder of mass M, length L and inner and outer radii\[{{R}_{1}}\]and\[{{R}_{2}}\]about the axis of the cylinder is
A)
\[M(R_{2}^{2}-R_{1}^{2})/2\]
done
clear
B)
\[\frac{M}{2{{L}^{2}}}(R_{2}^{4}-R_{1}^{4})\]
done
clear
C)
\[M(R_{2}^{2}+R_{1}^{2})/2\]
done
clear
D)
\[M{{({{R}_{2}}-{{R}_{1}})}^{2}}/2\]
done
clear
View Answer play_arrow
question_answer 6) A large number of bullets are fired in all the directions with the speed of 10 m/sec. The maximum area on the ground on which these bullets will spread is about\[(g=10\text{ }m/se{{c}^{2}})\]
A)
\[100\,{{m}^{2}}\]
done
clear
B)
\[250\,{{m}^{2}}\]
done
clear
C)
\[314\,{{m}^{2}}\]
done
clear
D)
\[506.5\text{ }{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 7) An aeroplane is flying horizontally with a velocity of 216 km/h and at a height of 1960 m. When it is vertically above a point A on the ground, a bomb is released from it. The bomb strikes the ground at point B. The distance AB is (ignoring air resistance)
A)
1200m
done
clear
B)
0.33 km
done
clear
C)
3.33km
done
clear
D)
33 km.
done
clear
View Answer play_arrow
question_answer 8) A ball is thrown with a kinetic energy E at an angle of\[45{}^\circ \]with the horizontal in the earths gravitational field. The change in its potential energy at the highest point of its flight with respect to the starting point will be
A)
\[+\frac{E}{\sqrt{2}}\]
done
clear
B)
\[+\frac{E}{2}\]
done
clear
C)
\[-\frac{E}{\sqrt{2}}\]
done
clear
D)
\[-\frac{E}{2}\]
done
clear
View Answer play_arrow
question_answer 9) A particle is projected vertically upward. The value of g is\[10\text{ }m/se{{c}^{2}}\]. If 10 and 16 seconds be the times at which it is at the same height while ascending and descending respectively. Then velocity of projection is
A)
260 m/sec
done
clear
B)
160 m/sec
done
clear
C)
130 m/sec
done
clear
D)
110 m/sec
done
clear
View Answer play_arrow
question_answer 10) Which of the following pairs does not have same dimension?
A)
angular momentum and Plancks constant
done
clear
B)
moment of inertia and moment of force
done
clear
C)
work and torque
done
clear
D)
impulse and momentum.
done
clear
View Answer play_arrow
question_answer 11) One-third of spring having force constant k is cut and mass m is suspended to it. If the frequency of oscillation of the original spring with the same mass is/then the frequency of oscillation in one-third of spring is
A)
\[\frac{1}{\sqrt{3}}f\]
done
clear
B)
\[\sqrt{3}f\]
done
clear
C)
\[2f\]
done
clear
D)
\[\frac{2}{\sqrt{3}}f\]
done
clear
View Answer play_arrow
question_answer 12) A copper ball is heated to\[100{}^\circ C\]. It cools to \[80{}^\circ C\]in 5 minutes and to\[65{}^\circ C\]in 10 minutes. The temperature of the surrounding is
A)
\[13{}^\circ C\]
done
clear
B)
\[15{}^\circ C\]
done
clear
C)
\[18{}^\circ C\]
done
clear
D)
\[20{}^\circ C\]
done
clear
View Answer play_arrow
question_answer 13) The ratio of specific heat at constant volume\[{{C}_{V}}\]for monoatomic gas to that of polyatomic gas i.e.\[{{C}_{V}}\](monoatomic)/\[{{C}_{V}}\](polyatomic) is
A)
1
done
clear
B)
5/6
done
clear
C)
5/3
done
clear
D)
½
done
clear
View Answer play_arrow
question_answer 14) The rate of difusion is
A)
faster in solids than in liquids and gases
done
clear
B)
faster in liquids than in solids and gases
done
clear
C)
equal to solids, liquids and gases
done
clear
D)
faster in gases than in liquids and solids.
done
clear
View Answer play_arrow
question_answer 15) A beaker of radius 6 cm is filled with mercury up to a height to 12 cm. Given that the density of mercury is\[13600\text{ }kg/{{m}^{3}},\]the force exerted by the mercury on the bottom of the beaker is approximately.
A)
184 N
done
clear
B)
1100N
done
clear
C)
1200 N
done
clear
D)
1300 N
done
clear
View Answer play_arrow
question_answer 16) The length of a needle floating on water is 2.5 cm. How much minimum force, in addition to the weight of the needle will be needed to lift the needle above the surface of water? Surface tension of water is\[7.2\times {{10}^{-4}}N/cm\].
A)
\[7.2\times {{10}^{-4}}N\]
done
clear
B)
\[18.0\times {{10}^{-4}}N\]
done
clear
C)
\[36.0\times {{10}^{-4}}N\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 17) A metal beam having coefficient of linear expansion\[\alpha \]Youngs modulus \[Y\], area of across section a and length L, is clamped at both ends. After cooling by\[t{}^\circ C,\]the force exerted in the clamp is
A)
\[YL\alpha t\]
done
clear
B)
\[Y\alpha a\]
done
clear
C)
\[YaL\alpha t\]
done
clear
D)
\[Ya\alpha t\]
done
clear
View Answer play_arrow
question_answer 18) The rotation period of an earth satellite close to the surface of the earth is 83 minutes. The satellite in an orbit at a distance of three times earth radii from 2 jts surface will be
A)
83 minutes
done
clear
B)
\[83\times \sqrt{8}\] minutes
done
clear
C)
664 minutes
done
clear
D)
249 minutes.
done
clear
View Answer play_arrow
question_answer 19) Two satellites A and B of equal mass are orbiting earth at altitudes R and 4R where R is the radius of the earth. The ratio of their kinetic energies is
A)
2
done
clear
B)
1/4
done
clear
C)
4
done
clear
D)
5/2
done
clear
View Answer play_arrow
question_answer 20) The ratio of the angular momenta associated with the earths rotation about its own axis and earths orbital motion is (radius of earth\[=6\times {{10}^{6}}\]m and mean earth-sun distance\[=1.5\times {{10}^{11}}m\])
A)
\[2.5\times {{10}^{-7}}:1\]
done
clear
B)
\[1:2.5\times {{10}^{-4}}\]
done
clear
C)
\[1:1\]
done
clear
D)
\[4\times {{10}^{-5}}:1\]
done
clear
View Answer play_arrow
question_answer 21) A 10-volt battery of negligible internal resistance is connected to\[50\,\Omega \],resistance coil. The heat energy produced in 1 hour is
A)
1714.3 calories
done
clear
B)
1530.5 calories
done
clear
C)
1050.0 calories
done
clear
D)
850.8 calories
done
clear
View Answer play_arrow
question_answer 22) Consider the following two facts about the free electron density in metals [A] it is different in different metals. [B] it is different at different temperatures in a metal. In view of the above, the Seebeck effect is caused
A)
due to both A and B
done
clear
B)
due to A but not due to B
done
clear
C)
due to B but not due to A
done
clear
D)
neither due to A nor due to B
done
clear
View Answer play_arrow
question_answer 23) When each of n identical resistance of resistance r are connected in series, the effective resistance is R. If these resistances are connected in parallel, the effective resistance of the combination will be
A)
\[R{{n}^{2}}\]
done
clear
B)
\[{{n}^{2}}/r\]
done
clear
C)
\[R{{r}^{2}}\]
done
clear
D)
\[{{r}^{2}}/R\]
done
clear
View Answer play_arrow
question_answer 24) A\[10\,\mu F\]capacitor is charged to a potential difference of 50 V and is connected to another uncharged capacitor in parallel. Now the common potential difference becomes 20 volt. The capacitance of second capacitor is
A)
\[15\,\mu F\]
done
clear
B)
\[30\,\mu F\]
done
clear
C)
\[20\,\mu F\]
done
clear
D)
\[10\,\mu F\]
done
clear
View Answer play_arrow
question_answer 25) Three wires whose lengths are in the ratio 2:6:18 and radii in the ratio\[1:2:3\]are connected in parallel. The ratio of the currents through them is
A)
\[1:\frac{1}{2}:\frac{1}{6}\]
done
clear
B)
\[\frac{1}{2}:\frac{2}{5}:\frac{4}{3}\]
done
clear
C)
\[3:3:4\]
done
clear
D)
\[3:4:3\]
done
clear
View Answer play_arrow
question_answer 26) A charge q is divided in two parts Q and\[(q-Q)\] If the magnitude of the force of repulsion between these two charges placed at certain separation is to be maximum the ratio \[q:(q-Q)\] is
A)
\[4:1\]
done
clear
B)
\[2:1\]
done
clear
C)
\[1:1\]
done
clear
D)
\[1:2\]
done
clear
View Answer play_arrow
question_answer 27) A hemispherical surface of the radius R is placed in a uniform electric field of strength E parallel to axis of the hemisphere. The electric flux through the curved surface of the hemisphere is
A)
zero
done
clear
B)
\[3\pi {{R}^{2}}E\]
done
clear
C)
\[2\pi {{R}^{2}}E\]
done
clear
D)
\[\pi {{R}^{2}}E\]
done
clear
View Answer play_arrow
question_answer 28) Each of the two conducting spheres of radius 5 cm and 10 cm is given a charge of\[15\mu C\]. After charging the spheres are connected by conducting wire. The charge on the smaller spheres after the connection is equal to.
A)
\[7.5\mu C\]
done
clear
B)
\[15\mu C\]
done
clear
C)
\[10\mu C\]
done
clear
D)
\[20\mu C\]
done
clear
View Answer play_arrow
question_answer 29) The velocity acquired by a particle of charge q and mass m moving through a potential difference of V volts will be
A)
\[2qV/m\]
done
clear
B)
\[\sqrt{2qm/V}\]
done
clear
C)
\[\sqrt{m/2qV}\]
done
clear
D)
\[\sqrt{2qV/m}\]
done
clear
View Answer play_arrow
question_answer 30) The difference between the apparent frequencies of whistle as perceived by an observer in rest during approach and recession of the train is 1%. If velocity of sound is 320 m/sec. The velocity of the train is
A)
5.7 km/hour
done
clear
B)
7.2 km/hour
done
clear
C)
10.3 km/hour
done
clear
D)
44.8 km/hour
done
clear
View Answer play_arrow
question_answer 31) An object of size 4 cm is placed 40 cm from a lens. An image is formed on a screen placed 80 cm on the other side of the lens. Which of the following statement is true?
A)
lens is convex, image is virtual and erect
done
clear
B)
lens is convex, image is real and inverted
done
clear
C)
lens is concave, image is real and inverted
done
clear
D)
lens is concave,, image is real and erect
done
clear
View Answer play_arrow
question_answer 32) An object is placed in front of a convex mirror at a distance equal to focal length of mirror. The magnification of the object is
A)
zero
done
clear
B)
\[\infty \]
done
clear
C)
\[\frac{1}{2}\]
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 33) The intensity ratio of the maxima and minima in an interference pattern produced by two coherent sources of light is 9 1. The intensities of the used light sources are in ratio
A)
\[3:1\]
done
clear
B)
\[4:1\]
done
clear
C)
\[9:1\]
done
clear
D)
\[10:1\]
done
clear
View Answer play_arrow
question_answer 34) In Youngs double slit experiment if separation between the slits is halved, the fringe width is
A)
halved
done
clear
B)
doubled
done
clear
C)
unchanged
done
clear
D)
quadrupled
done
clear
View Answer play_arrow
question_answer 35) Who produced electromagnetic waves first?
A)
Marconi
done
clear
B)
Maxwell
done
clear
C)
J.C. Bose
done
clear
D)
Hertz
done
clear
View Answer play_arrow
question_answer 36) A series RLC circuit is connected to 230V source where\[R=40\,\Omega ,L=2H,C=50\mu F\]. The amplitude of current at resonance is
A)
6.25 A
done
clear
B)
5.25 A
done
clear
C)
5.75 A
done
clear
D)
8.13 A
done
clear
View Answer play_arrow
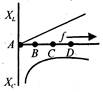
question_answer 37)
The variation of inductive reactance\[({{X}_{L}})\]and capacitive reactance \[({{X}_{c}})\]is shown in figure. The condition of resonance occurs at points
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
View Answer play_arrow
question_answer 38) A magnet makes 30 vibrations per minute at a place, How many vibrations will it make per minute if it is remagnetized so that its pole strength is doubled
A)
\[\sqrt{{}}600\]
done
clear
B)
\[\sqrt{{}}1200\]
done
clear
C)
\[\sqrt{{}}1800\]
done
clear
D)
\[\sqrt{{}}445\]
done
clear
View Answer play_arrow
question_answer 39) A current of 5 amp flows in a circular loop of radius 5 cm. The loop is placed in a uniform magnetic field of 5 tesia such that plane of loop is perpendicular to the direction of magnetic field. The net magnetic force acting on the loop if there are 5 turns, is
A)
zero
done
clear
B)
\[\frac{{{\mu }_{0}}}{4\pi }\times 6.2\]newton
done
clear
C)
12.5 newton
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 40) A wire of length L carrying a current\[I\]ampere is bent into from of a circle. The magnitude of magnetic moment is
A)
\[IL\]
done
clear
B)
\[{{I}^{2}}L\]
done
clear
C)
\[I{{L}^{2}}\]
done
clear
D)
\[I{{L}^{2}}/4\pi \]
done
clear
View Answer play_arrow
question_answer 41) Two stars A and B at equal surface temperature. Star A has surface area four times that of B. The ratio in the luminosities of A and B will be
A)
\[\begin{matrix} 1 & 16 \\ \end{matrix}\]
done
clear
B)
\[\begin{matrix} 1 & 4 \\ \end{matrix}\]
done
clear
C)
\[\begin{matrix} 16 & 1 \\ \end{matrix}\]
done
clear
D)
\[\begin{matrix} 4 & 1 \\ \end{matrix}\]
done
clear
View Answer play_arrow
question_answer 42) Hole and electron mobilities of semiconductor are 0.05 and\[0.15\,{{m}^{2}}{{V}^{-1}}s\]. An electric field of strength\[4\text{ }Vc{{m}^{-1}}\]is applied to it. The ratio of drift velocities of electron and hole is
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 43)
The output of the following logic circuit is
A)
\[Y=\overline{A}\div \overline{B}\]
done
clear
B)
\[Y=\overline{A}.\overline{B}\]
done
clear
C)
\[Y=\overline{A.B}\]
done
clear
D)
\[Y=\overline{A+B}\]
done
clear
View Answer play_arrow
question_answer 44) In a (BJT) bipolar junction transistor
A)
\[\alpha =\beta \]
done
clear
B)
\[\beta <\alpha \]
done
clear
C)
\[\beta =\frac{\alpha }{1+\alpha }\]
done
clear
D)
\[\beta =\frac{\alpha }{1-\alpha }\]
done
clear
View Answer play_arrow
question_answer 45) Forbidden energy gap for diamond is of the order of
A)
0.1 eV
done
clear
B)
1.0 eV
done
clear
C)
6 eV
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 46) In the gamma decay of a nucleus
A)
both the atomic number and the atomic mass number increase by one unit
done
clear
B)
both the atomic number and atomic mass number decrease by one unit
done
clear
C)
the atomic number decrease by one unit
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 47) The mass defect in a nuclear reaction is 0.25 percent. The energy liberated by fusion of one kg matter will be
A)
\[2.25\times {{10}^{7}}J\]
done
clear
B)
\[2.25\times {{10}^{14}}J\]
done
clear
C)
\[6.75\times {{10}^{10}}J\]
done
clear
D)
\[4.5\times {{10}^{7}}J\]
done
clear
View Answer play_arrow
question_answer 48) Which one of the following element has highest 55, binding energy per nucleon?
A)
He
done
clear
B)
Fe
done
clear
C)
Th
done
clear
D)
U
done
clear
View Answer play_arrow
question_answer 49) When the electrons in hydrogen atom jumps from the orbit with\[n=2\]to\[n=1\]the frequency of emitted radiation is o. If the electron jumps from the orbit with\[n=3\]to\[n=2\]frequency of the emitted is
A)
\[\frac{5}{27}\upsilon \]
done
clear
B)
\[\frac{5}{36}\upsilon \]
done
clear
C)
\[\frac{3}{4}\upsilon \]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 50) An X-ray tube is operated at 1 kV. The kinetic energy of electrons hitting the anticathode is
A)
\[\ge 18KeV\]
done
clear
B)
\[=18\text{ }keV\]
done
clear
C)
\[\le 18KeV\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 51) Which is correct increasing order of their tendency to from\[{{M}^{3-}}\]ion?
A)
\[Bi>Sb>As>P>N\]
done
clear
B)
\[Bi<Sb<As<P<N\]
done
clear
C)
\[N<P<Sb<Bi<As\]
done
clear
D)
\[Bi>Sb\tilde{\ }N\tilde{\ }P>As\]
done
clear
View Answer play_arrow
question_answer 52) Which is first rare gas compound discovered in 1962?
A)
\[Xe{{F}_{2}}\]
done
clear
B)
\[Xe{{O}_{4}}\]
done
clear
C)
\[XePt{{F}_{6}}\]
done
clear
D)
\[Xe{{F}_{6}}\]
done
clear
View Answer play_arrow
question_answer 53) A radio isotope decays of such a rate that after 100 minutes only eight of the original amount remains, the half life of radio isotope is
A)
31.3 min
done
clear
B)
32.3 min
done
clear
C)
33.3 min
done
clear
D)
34.3 min
done
clear
View Answer play_arrow
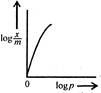
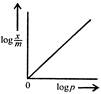
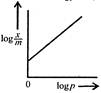
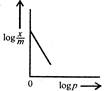
question_answer 54) Freundlich Adsorption Isotherm\[x/m=k{{p}^{1/m}}\]may be graphically represented as
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 55) Which polymer is used for making magnetic recording tapes?
A)
dacron
done
clear
B)
acrilan
done
clear
C)
glyptal
done
clear
D)
bakelite
done
clear
View Answer play_arrow
question_answer 56) Amino acids, which build up proteins, have both the carboxylic and amino groups. These amino acids are
A)
\[\alpha -\]amino acids
done
clear
B)
\[\beta -\]amino acids
done
clear
C)
\[\gamma -\]amino acids
done
clear
D)
position of amino and carboxylic groups are unspecified
done
clear
View Answer play_arrow
question_answer 57) Which of the following amino acids is basic in nature?
A)
Serine
done
clear
B)
Glutamine
done
clear
C)
Arginine
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 58) \[\underset{\begin{smallmatrix} | \\ C{{H}_{2}}OOCR \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{2}}OCR \\ | \end{smallmatrix}}{\mathop{CHOOCR}}}\,\xrightarrow[Hydrolysis]{Enzyne}\underset{\begin{smallmatrix} | \\ C{{H}_{2}}OH \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{2}}OH \\ | \end{smallmatrix}}{\mathop{CHOH}}}\,+\underset{\begin{smallmatrix} + \\ R\,COOH \end{smallmatrix}}{\overset{\begin{smallmatrix} RCOOH \\ + \end{smallmatrix}}{\mathop{RCOOH}}}\,\] The enzyme used in the above reaction is
A)
amylase
done
clear
B)
lactase
done
clear
C)
lipase
done
clear
D)
invertase
done
clear
View Answer play_arrow
question_answer 59)
The general structure of penicillin is
A)
done
clear
B)
done
clear
C)
\[-C{{H}_{2}}-CH=CH-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
D)
\[(C{{H}_{3}}){{(C{{H}_{2}})}_{6}}-\]
done
clear
View Answer play_arrow
question_answer 60) The ionization energy of elements in period increases as we proceed from left to right because
A)
the number of electrons increases successively
done
clear
B)
the number of neutrons increases successively
done
clear
C)
the number protons increases successively
done
clear
D)
electrons being filled in orbitals with same principal quantum number repel each-other
done
clear
View Answer play_arrow
question_answer 61) A corked flask containing boiling water and its vapour is allowed to cool down to room temperature. If water and its vapour as a whole is the system then
A)
\[\Delta S>0\]and \[-\Delta H=\]loss of heat in cooling
done
clear
B)
\[\Delta S<0\] and\[-\Delta H=\]loss of heat in cooling
done
clear
C)
\[\Delta S>0\]and \[-\Delta E=\]loss of heat in cooling
done
clear
D)
\[\Delta S<0\]and\[-\Delta E=\]loss of heat in cooling
done
clear
View Answer play_arrow
question_answer 62) At\[0{}^\circ K,\](i)\[{{C}^{12}}\]and (ii) a mixture of\[{{C}^{12}}\]and\[{{C}^{14}}\] will
A)
both have zero entropy
done
clear
B)
both have positive entropy
done
clear
C)
have equal entropies per mole
done
clear
D)
have unequal entropies
done
clear
View Answer play_arrow
question_answer 63) At any instant during the reaction \[Zn+C{{u}^{++}}\to Z{{n}^{++}}\] \[+Cu\]occurring in an open beaker at temperature T
A)
\[\Delta G{}^\circ =-RT\]in\[\frac{[Z{{n}^{++}}].[Cu]}{[Zn].[C{{u}^{++}}]}\]
done
clear
B)
\[-\Delta G=\]work available from the reaction
done
clear
C)
\[\Delta G=0\]
done
clear
D)
\[\Delta G<0\]
done
clear
View Answer play_arrow
question_answer 64) The first order rate constant is expressed as \[K=\frac{2.303}{t}\log ({{C}_{0}}/{{C}_{t}})\]while \[K\]depends on
A)
time \[(t)\]
done
clear
B)
initial concentration of the reactant \[({{C}_{0}})\]
done
clear
C)
the ratio \[{{C}_{0}}/{{C}_{t}}\]
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 65) The general reaction \[R-X\xrightarrow[{}]{aq\,H{{O}^{-}}}ROH+{{X}^{O-}}\] is expected to follow decreasing order of reactivity as in
A)
\[t-Bui>t-BuBr>t-BuCl>t-Bu-F\]
done
clear
B)
\[t-BuF>t-BuCl>t-BuBr>t-Bul\]
done
clear
C)
\[t-BuBr>t-BuCl>t-Bul>t-BuF\]
done
clear
D)
\[t-BuF>t-BuCl>t-Bul>t-BuBr\] (\[t-Bu=\]tertiary Butyl group)
done
clear
View Answer play_arrow
question_answer 66) Under basic conditions which one suffers elimination the most?
A)
\[{{(C{{H}_{3}})}_{3}}CCl\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}-C{{H}_{2}}-Cl\]
done
clear
D)
done
clear
View Answer play_arrow
question_answer 67) The percentage strength (in g \[litr{{e}^{-1}}\]) of 10 ml of 20 vol. solution of\[{{H}_{2}}{{O}_{2}}\]is
A)
220.4
done
clear
B)
110.2
done
clear
C)
150.5
done
clear
D)
60.7
done
clear
View Answer play_arrow
question_answer 68) Which of the following is low boiling liquid?
A)
HF
done
clear
B)
\[HCl\]
done
clear
C)
\[HBr\]
done
clear
D)
\[HI\]
done
clear
View Answer play_arrow
question_answer 69) Which of the following statement about water is wrong?
A)
it can act as a base
done
clear
B)
it can act as an acid
done
clear
C)
it can act both as an acid and as a base
done
clear
D)
it can act neither as acid nor as base
done
clear
View Answer play_arrow
question_answer 70) The brown ring formation in the test for nitrate is due to
A)
\[{{[Fe({{H}_{2}}O){{(NO)}_{5}}]}^{2+}}\]
done
clear
B)
\[{{[Fe{{({{H}_{2}}O)}_{5}}(NO)]}^{2+}}\]
done
clear
C)
\[Fe{{(N{{O}_{3}})}_{3}}\]
done
clear
D)
\[{{[Fe{{({{H}_{2}}O)}_{5}}(N{{O}_{2}})]}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 71) The Markownikoffs addition rule is applicable for the reaction between
A)
Symmetrical alkene\[+\]Symmetrical reagent
done
clear
B)
Symmetrical alkene\[+\]Unsymmetrical reagent
done
clear
C)
Unsymmetrical alkene\[+\]Symmetrical reagent
done
clear
D)
Unsymmetrical alkene\[+\]Unsymmetrical reagent
done
clear
View Answer play_arrow
question_answer 72) An alkene, \[{{C}_{6}}{{H}_{12}}\]on ozonolysis gives acetone and propanal. It has the structure
A)
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}-CH=CH-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 73) An unknown compound A has a molecular formula\[{{C}_{4}}{{H}_{6}},\]when A is treated with an excess of\[B{{r}_{2}},\]a new substance B with formula \[{{C}_{4}}{{H}_{6}}B{{r}_{4}}\]is formed. A forms a white precipitate with ammoniacal silver nitrate solution. A may be
A)
Butyne-1
done
clear
B)
Butyne - 2
done
clear
C)
Butene - 1
done
clear
D)
Butene - 2.
done
clear
View Answer play_arrow
question_answer 74) In Lassaigne test a violet colour obtained indicates the presence of
A)
nitrogen
done
clear
B)
sulphur
done
clear
C)
halogen
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 75) Hereditary characteristic are passed on from parents to children through
A)
Gametes
done
clear
B)
Genes
done
clear
C)
Mutants
done
clear
D)
Enzymes
done
clear
View Answer play_arrow
question_answer 76) Which of the following covalent bonds has highest ionic character?
A)
\[H-F\]
done
clear
B)
\[H-Cl\]
done
clear
C)
\[H-Br\]
done
clear
D)
\[H-\text{I}\]
done
clear
View Answer play_arrow
question_answer 77) The isomeric forms known for xylene are
A)
2
done
clear
B)
3
done
clear
C)
4
done
clear
D)
5
done
clear
View Answer play_arrow
question_answer 78) The shape of benzene is
A)
linear
done
clear
B)
tetrahedral
done
clear
C)
planar
done
clear
D)
pyramidal
done
clear
View Answer play_arrow
question_answer 79) The hybridization of carbon in\[C-C\]single bond of \[CH=CH-C\equiv CH\]is
A)
\[sp-sp\]
done
clear
B)
\[sp-s{{p}^{2}}\]
done
clear
C)
\[s{{p}^{2}}-sp\]
done
clear
D)
\[s{{p}^{2}}-s{{p}^{2}}\]
done
clear
View Answer play_arrow
question_answer 80) An ideal solution shows a vapour pressure of 80 torr when the mole-fraction of the solute is 0.2. At the same temperature, the vapour pressure of the pure solvent should be
A)
64 torr
done
clear
B)
80 torr
done
clear
C)
100 torr
done
clear
D)
400 torr
done
clear
View Answer play_arrow
question_answer 81) For the reactions (I) \[{{H}_{2}}(g)+{\scriptstyle{}^{1}/{}_{2}}{{O}_{2}}(g)\xrightarrow[{}]{{}}{{H}_{2}}O(g)+{{x}_{1}}kJ\] (II) \[{{H}_{2}}(g)+{\scriptstyle{}^{1}/{}_{2}}{{O}_{2}}(g)\xrightarrow[{}]{{}}{{H}_{2}}O(g)+{{x}_{2}}kJ\] Which one is correct?
A)
\[{{x}_{1}}<{{x}_{2}}\]
done
clear
B)
\[{{x}_{1}}={{x}_{2}}\]
done
clear
C)
\[{{x}_{1}}>{{x}_{2}}\]
done
clear
D)
\[{{x}_{1}}-{{x}_{2}}=0\]
done
clear
View Answer play_arrow
question_answer 82) For the reaction\[2S{{O}_{3}}(g)\xrightarrow[{}]{{}}2S{{O}_{2}}(g)+{{O}_{2}}(g)\]the entropy change\[(\Delta S)\]is
A)
\[\Delta S>0\]
done
clear
B)
\[\Delta S<0\]
done
clear
C)
\[\Delta S=0\]
done
clear
D)
\[\Delta S<\frac{\Delta H}{T}\]
done
clear
View Answer play_arrow
question_answer 83) The value of\[\Delta S\]for the reaction\[Zn+C{{u}^{++}}=Z{{n}^{++}}\]\[+Cu\]conducted reversibly in galvanic cell is\[Q/T\]at temperature T. Its value for the same reaction will be
A)
\[Q/T\] if conducted irreversibly
done
clear
B)
above\[Q/T\]if conducted irreversibly
done
clear
C)
\[\Delta H/T\]if conducted irreversibly
done
clear
D)
\[Q/T\]at temperature T
done
clear
View Answer play_arrow
question_answer 84) Which among following cannot be a Bronsted acid?
A)
\[NH_{4}^{+}\]
done
clear
B)
\[HSO_{3}^{-}\]
done
clear
C)
\[{{H}_{2}}O\]
done
clear
D)
\[S{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 85) Addition of sodium acetate to aqueous \[C{{H}_{3}}COOH\]solution
A)
increases both pH and % dissociation of \[C{{H}_{3}}COOH\]
done
clear
B)
decreases both pH and % dissociation of \[C{{H}_{3}}COOH\]
done
clear
C)
increases pH and decreases % dissociation of\[C{{H}_{3}}COOH\]
done
clear
D)
decreases pH and increase% dissociation of \[C{{H}_{3}}COOH\]
done
clear
View Answer play_arrow
question_answer 86) Oxidation numbers of carbon in\[C{{H}_{2}}C{{l}_{2}},C{{O}_{2}}\] and\[CO_{3}^{2-}\]are respectively
A)
\[0,+4,+4\]
done
clear
B)
\[+2,+2,-2\]
done
clear
C)
\[+4,+4,+4\]
done
clear
D)
\[-4,+4,+2\]
done
clear
View Answer play_arrow
question_answer 87) Oxidation number of iron in\[{{[Fe{{(CN)}_{6}}]}^{4-}}\]is
A)
\[+2\]
done
clear
B)
\[+3\]
done
clear
C)
\[-3\]
done
clear
D)
\[-4\]
done
clear
View Answer play_arrow
question_answer 88) Which one has maximum ionic character?
A)
\[Pb{{F}_{2}}\]
done
clear
B)
\[PbC{{l}_{2}}\]
done
clear
C)
\[PbB{{r}_{2}}\]
done
clear
D)
\[Pb{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 89) Zinc reacts with dilute\[HN{{O}_{3}}\]to give
A)
\[{{N}_{2}}O\]
done
clear
B)
\[N{{O}_{2}}\]
done
clear
C)
\[{{N}_{2}}{{O}_{4}}\]
done
clear
D)
\[N{{H}_{4}}N{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 90) The\[{{K}_{w}}\]for autoprotolysis of water at 298K \[2{{H}_{2}}{{O}_{(l)}}\rightleftharpoons {{H}_{3}}{{O}^{+}}_{(aq)}+O{{H}^{-}}_{(aq)}\]is
A)
\[{{10}^{-14}}mo{{l}^{2}}{{L}^{-2}}\]
done
clear
B)
\[{{10}^{-14}}mo{{l}^{-1}}{{L}^{-1}}\]
done
clear
C)
\[{{10}^{-4}}mol{{L}^{-1}}\]
done
clear
D)
\[{{10}^{-4}}mo{{l}^{2}}{{L}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 91) Which one of the following is most likely to form an ionic bond?
A)
H and \[O\]
done
clear
B)
H and \[Cl\]
done
clear
C)
H and N
done
clear
D)
Na and \[Cl\]
done
clear
View Answer play_arrow
question_answer 92) The conjugate base of\[O{{H}^{-}}\]is
A)
\[{{H}^{+}}\]
done
clear
B)
\[{{H}_{2}}O\]
done
clear
C)
\[OH_{2}^{-}\]
done
clear
D)
\[{{O}^{2-}}\]
done
clear
View Answer play_arrow
question_answer 93) \[{{K}_{3}}Fe{{(CN)}_{6}}+\overline{e}\xrightarrow[{}]{{}}{{K}_{4}}Fe{{(CN)}_{6}}\]is known as
A)
ioriization
done
clear
B)
oxidation
done
clear
C)
reduction
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 94) If 100 g iron powder (at. wt. of\[Fe=56\]) is heated with 32 g of sulphur, (at wt. of\[S=32\]) to prepare one mol of iron sulphide how much iron powder will remain unreacted?
A)
4 g
done
clear
B)
32 g
done
clear
C)
44 g
done
clear
D)
Nil
done
clear
View Answer play_arrow
question_answer 95) One mole of ideal gas is filled in 2 litre flask at \[0{}^\circ C,\]the pressure of the gas will be
A)
1 atm
done
clear
B)
11.2 atm
done
clear
C)
22.4 atm
done
clear
D)
44.8 atm
done
clear
View Answer play_arrow
question_answer 96) Translational kinetic energy for 2 moles of gas at\[27{}^\circ C\]is
A)
\[7.48\times {{10}^{3}}J\]
done
clear
B)
\[6.48\times {{10}^{3}}J\]
done
clear
C)
\[5.48\times {{10}^{3}}J\]
done
clear
D)
\[4.48\times {{10}^{3}}J\]
done
clear
View Answer play_arrow
question_answer 97) The molecular mass of an unknown gas that diffuses twice as fast as oxygen is
A)
\[0.032\text{ }kg\text{ }mo{{l}^{-1}}\]
done
clear
B)
\[0.016\text{ }kg\text{ }mo{{l}^{-1}}\]
done
clear
C)
\[0.004\text{ }kg\text{ }mo{{l}^{-1}}\]
done
clear
D)
\[0.008\text{ }kg\text{ }mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 98) Which of the following bonds will be have highest ionic character?
A)
\[H-F\]
done
clear
B)
\[H-Cl\]
done
clear
C)
\[H-Br\]
done
clear
D)
\[H-I\]
done
clear
View Answer play_arrow
question_answer 99) Which of the following expression represents the energy of electron in a hydrogen atom?
A)
\[E=\frac{2{{\pi }^{2}}m{{e}^{4}}}{{{h}^{2}}}\frac{1}{{{n}^{2}}}\]
done
clear
B)
\[E=\frac{4{{\pi }^{2}}m{{e}^{2}}}{{{h}^{2}}}\frac{1}{{{n}^{2}}}\]
done
clear
C)
\[E=-\frac{2{{\pi }^{2}}m{{e}^{4}}}{{{h}^{2}}}\frac{1}{{{n}^{2}}}\]
done
clear
D)
\[E=-\frac{4{{\pi }^{2}}m{{e}^{2}}}{{{h}^{2}}}\frac{1}{{{n}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 100) For a reaction in a galvanic cell the value of\[(-\Delta G)\]at temperature T is not necessarily equal to
A)
\[nF{{E}_{cell}}\]
done
clear
B)
RT In k
done
clear
C)
\[T\Delta S-\Delta H\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 101) A bacterial cell divides in every minute. It takes one hour to fill a cup. How much time it will take to fill the half cup?
A)
59 minutes
done
clear
B)
30 minutes
done
clear
C)
29 minutes
done
clear
D)
58 minutes.
done
clear
View Answer play_arrow
question_answer 102) The role of the oxidative pentose phosphate pathway is to generate reducing potential in the form of
A)
NADPH only
done
clear
B)
NADH only
done
clear
C)
FADH only
done
clear
D)
FADH and NADH.
done
clear
View Answer play_arrow
question_answer 103) Five gram mole of glucose, on complete oxidation releases
A)
3430 k. calories of energy
done
clear
B)
343 k. calories of energy
done
clear
C)
3030 k. calories of energy
done
clear
D)
430 k. calories of energy.
done
clear
View Answer play_arrow
question_answer 104) Conversion of phosphoglyceraldehyde to 1, 3- biphosphogly eerie acid is a
A)
priming reaction only
done
clear
B)
redox reaction only
done
clear
C)
priming and redox reaction only
done
clear
D)
substrate level phosphorylation reaction.
done
clear
View Answer play_arrow
question_answer 105) An eukaryotic cell can synthesise only 38 ATP instead of 40 ATP if the protons of cytosolic NADH-HhT are transported into mitochondrial matrix by
A)
malate - aspartate shuttle
done
clear
B)
glycerol phosphate shuttle
done
clear
C)
ATPase
done
clear
D)
permease.
done
clear
View Answer play_arrow
question_answer 106) The membrane surrounding the vacuole is known as
A)
cell membrane
done
clear
B)
plasmalemma
done
clear
C)
tonoplast
done
clear
D)
chromoplast.
done
clear
View Answer play_arrow
question_answer 107) The largest cell organelles in the cell are
A)
plastids
done
clear
B)
golgi bodies
done
clear
C)
mitochondria
done
clear
D)
chromosomes.
done
clear
View Answer play_arrow
question_answer 108) The organelles for photorespiration are
A)
lysosomes
done
clear
B)
glyoxysomes
done
clear
C)
peroxisomes
done
clear
D)
ribosome.
done
clear
View Answer play_arrow
question_answer 109) The active transport of substances across the plasma membrane is done with the help of
A)
diffusion
done
clear
B)
metabolic energy
done
clear
C)
electrochemical gradient
done
clear
D)
both [b] & [c].
done
clear
View Answer play_arrow
question_answer 110) Molecules moves inside and outside of living cells by
A)
osmosis only
done
clear
B)
diffusion only
done
clear
C)
osmosis and diffusion
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 111) Deforestation has caused
A)
more floods and increase of temperature
done
clear
B)
more drought and decrease of temperature
done
clear
C)
shortage of food products
done
clear
D)
soil erosion and loss of wild life.
done
clear
View Answer play_arrow
question_answer 112) Which of the following plant is an endangered species in India?
A)
Lycopodium sp.
done
clear
B)
Pinus roxburghii
done
clear
C)
Hydrilla
done
clear
D)
Cerdus deodar a.
done
clear
View Answer play_arrow
question_answer 113) The region of earth comprising water forms
A)
hydrosphere
done
clear
B)
lithosphere
done
clear
C)
biosphere
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 114) Which one of the following is the food chain operating in ponds, lakes or sea?
A)
grass\[\to \]insects\[\to \]frog\[\to \]birds
done
clear
B)
\[\underset{\begin{smallmatrix} (phytopi- \\ anktons) \end{smallmatrix}}{\mathop{algae}}\,\to \underset{(zooplanktons)}{\mathop{small\text{ }animals}}\,\to fish\to big\text{ }fish\]
done
clear
C)
grass\[\to \]dear\[\to \]lion
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 115) Reserve food in the form of floridean starch and the soluble sugar-floridoside is found in
A)
phaeophyta
done
clear
B)
chlorophyta
done
clear
C)
cyanophyta
done
clear
D)
rhodophyta.
done
clear
View Answer play_arrow
question_answer 116) A plant showing vascular tissue but not producing seed belongs to
A)
gymnosperms
done
clear
B)
pteridophytes
done
clear
C)
bryophytes
done
clear
D)
angiosperms.
done
clear
View Answer play_arrow
question_answer 117) Mutualistic symbiosis of a fungus with the roots of a higher plant is termed as
A)
protists
done
clear
B)
mycorrhiza
done
clear
C)
lichens
done
clear
D)
moulds.
done
clear
View Answer play_arrow
question_answer 118) Which of the following fungi is heteroecious?
A)
Ustilago
done
clear
B)
Puccinia
done
clear
C)
Albugo
done
clear
D)
Phytophthora.
done
clear
View Answer play_arrow
question_answer 119) Citric acid is produced by fermentation of cane molasses (sucrose) by
A)
Penicillium notanum
done
clear
B)
Rhizopus spp
done
clear
C)
Aspergillus niger
done
clear
D)
Mucor spp.
done
clear
View Answer play_arrow
question_answer 120) Gemini viruses are plant viruses with
A)
circular single stranded RNA
done
clear
B)
circular single stranded DNA
done
clear
C)
linear single stranded DNA
done
clear
D)
linear single stranded RNA.
done
clear
View Answer play_arrow
question_answer 121) Of the total potential energy of glucose only about 15% is conserved as the potential energy of ATP and about 50% is lost as heat during alcoholic fermentation. The remaining energy is accounted for by the
A)
\[C{{O}_{2}}\]produced
done
clear
B)
NADH produced
done
clear
C)
growth and reproduction of yeast cells
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 122) Chlorophyll solution appears green in transmitted light but in reflected light it appears red because
A)
of phenomenon of fluorescence
done
clear
B)
it reflect red light
done
clear
C)
both of the above
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 123) Casparian strips are characteristic of
A)
cortex
done
clear
B)
epidermis
done
clear
C)
pith
done
clear
D)
endodermis.
done
clear
View Answer play_arrow
question_answer 124) Secondary phloem remains functional generally
A)
for one year
done
clear
B)
for less than one year
done
clear
C)
for many year
done
clear
D)
as long as the plant is alive.
done
clear
View Answer play_arrow
question_answer 125) Intergeneric crosses are rarely successful under natural condition. Which of the following methods may be beneficial in this regard?
A)
embryo culture
done
clear
B)
tissue culture
done
clear
C)
anther culture
done
clear
D)
protoplast culture.
done
clear
View Answer play_arrow
question_answer 126) During megasporogenesis after first phase of meiosis the micropylar dyad cell degenerates while the chalazal one undergoes 2nd phase or meiosis producing 2 megaspores which take part in the development of female gametophyte. This type of female gametophyte development is known as
A)
Adoxa type
done
clear
B)
Allium type
done
clear
C)
Endymion type
done
clear
D)
Oenothera type.
done
clear
View Answer play_arrow
question_answer 127) The cell organelle which is found between the cell wall and cell membrane is
A)
lysosome
done
clear
B)
microsome
done
clear
C)
lomasome
done
clear
D)
middle lamella.
done
clear
View Answer play_arrow
question_answer 128) Pollution is an unavoidable consequence of
A)
industrialisation
done
clear
B)
population
done
clear
C)
urbanisation
done
clear
D)
deforestation.
done
clear
View Answer play_arrow
question_answer 129) Which of the following is a chlorofluorocarbon?
A)
\[CFS{{O}_{2}}\]
done
clear
B)
\[C{{F}_{2}}C{{l}_{2}}\]
done
clear
C)
\[FClC{{O}_{2}}\]
done
clear
D)
\[Cl{{F}_{2}}C\]
done
clear
View Answer play_arrow
question_answer 130) The ratio 1 1 1 1 is expected as a result of
A)
monohybrid cross
done
clear
B)
monohybrid back cross
done
clear
C)
dihybrid cross
done
clear
D)
dihybrid test cross.
done
clear
View Answer play_arrow
question_answer 131) During RNA transcription, unwinding of DNA is performed by
A)
topoisomerase
done
clear
B)
helicase
done
clear
C)
RNA polymerase
done
clear
D)
ligase.
done
clear
View Answer play_arrow
question_answer 132) Macroelements must generally be present in the plant tissues in concentration of at least
A)
0.001 mg/g dry matter
done
clear
B)
0.01 mg/g dry matter
done
clear
C)
0.1 mg/g dry matter
done
clear
D)
1.0 mg/g dry matter.
done
clear
View Answer play_arrow
question_answer 133) Uptake of mineral ions against concentration gradient is called
A)
active absorption
done
clear
B)
passive absorption
done
clear
C)
negative absorption
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 134) Dahlia and Asparagus possess
A)
stilt roots
done
clear
B)
fusiform roots
done
clear
C)
tuberous roots
done
clear
D)
fasciculated roots.
done
clear
View Answer play_arrow
question_answer 135) The first electron acceptor in cyclic photo- phosphorylation is
A)
\[Fd\]
done
clear
B)
\[PQ\]
done
clear
C)
\[PC\]
done
clear
D)
Cyt.b.
done
clear
View Answer play_arrow
question_answer 136) Cuticle is
A)
acellular layer on epidermis, which is visible and found in most organ
done
clear
B)
acellular layer on epidermis which is visible or invisible, found in all organs
done
clear
C)
acellular layer on epidermis of dead cells
done
clear
D)
a layer on epidermis which is dead and visible or invisible.
done
clear
View Answer play_arrow
question_answer 137) Where as chlorosis is produced in leaves due to deficiency of\[Fe,Mg,Mn,N\]or S. Of these essential element those that are exclusive constituents of chlorophyll molecules are
A)
Fe and S
done
clear
B)
N and S
done
clear
C)
Mg and S
done
clear
D)
Mg and N.
done
clear
View Answer play_arrow
question_answer 138) P700 is named because
A)
it is 700 times more efficient than other pigment molecules
done
clear
B)
its absorption and action spectra show peaks at 700nm
done
clear
C)
it is rendered ineffective at wave length above 700nm
done
clear
D)
it is a unit consisting of 700 chorophyll molecules.
done
clear
View Answer play_arrow
question_answer 139) Two enzymes common to EMP pathway and \[{{C}_{3}}\]cycle are
A)
aldolase and triose phosphate isomerase
done
clear
B)
aldolase and enolase
done
clear
C)
cytochrome oxidase and enolase
done
clear
D)
phosphoglyceromutase and triose phosphate isomerase.
done
clear
View Answer play_arrow
question_answer 140) The quickest method of plant breeding is
A)
introduction
done
clear
B)
selection
done
clear
C)
hybridization
done
clear
D)
mutation breeding
done
clear
View Answer play_arrow
question_answer 141) Mutation in which thymine is replaced by cytosine is called
A)
transition
done
clear
B)
transversion
done
clear
C)
missense
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 142) Which of the following organelle is related with genetic engineering?
A)
plastids
done
clear
B)
plasmids
done
clear
C)
mitochondria
done
clear
D)
ribosomes.
done
clear
View Answer play_arrow
question_answer 143) Basic elements of life are
A)
\[C,H,O,N\]
done
clear
B)
\[C,P,H,S\]
done
clear
C)
\[H,O,N,S\]
done
clear
D)
\[P,C,O,N\]
done
clear
View Answer play_arrow
question_answer 144) Discovery of fossils of cyanobacteria indicates that life originated sometime
A)
prior to 4600 million years ago
done
clear
B)
between 4600 and 3600 million years ago
done
clear
C)
more than 3600 million years ago
done
clear
D)
during some other period.
done
clear
View Answer play_arrow
question_answer 145) The shape of the chromosomes is usually observed at
A)
metaphase 1 of the meiosis
done
clear
B)
anaphase 1 of meiosis
done
clear
C)
metaphase of mitosis
done
clear
D)
anaphase of mitosis.
done
clear
View Answer play_arrow
question_answer 146) The small segment of DNA which helps to detect the presence of a gene or a long DNA sequence in biological system is termed as
A)
DNA walker
done
clear
B)
\[cDNA\]
done
clear
C)
DNA clone
done
clear
D)
DNA probe.
done
clear
View Answer play_arrow
question_answer 147) When one gene dominates the other non-allelic gene, the phenomenon is known as
A)
dominance
done
clear
B)
tolerance
done
clear
C)
homeostasis
done
clear
D)
epistasis
done
clear
View Answer play_arrow
question_answer 148) The distance between two nucleotides in DNA is
A)
\[34\text{ }{{A}^{o}}\]
done
clear
B)
\[3.4\text{ }{{A}^{o}}\]
done
clear
C)
\[0.34\text{ }{{A}^{o}}\]
done
clear
D)
\[\text{20 }{{A}^{o}}\]
done
clear
View Answer play_arrow
question_answer 149) The start signal in mRNA for protein synthesis is
A)
AUG
done
clear
B)
UAG
done
clear
C)
GUA
done
clear
D)
UGA.
done
clear
View Answer play_arrow
question_answer 150) Glycolysis consists of
A)
ten enzymatic reactions that convert a six- carbon molecule of four-carbon pyruvate and result in a net gain of 4 ATP molecules
done
clear
B)
eight enzymatic reactions that convert a six-carbon molecule to four-carbon pyruvate and result in a net gain of 4 ATP molecules
done
clear
C)
ten enzymatic reactions that convert a six- carbon molecules to three-carbon pyruvate and result in a net gain of 4 ATP molecules
done
clear
D)
eight enzymatic reactions that convert a six- carbon molecules to three-carbon pyruvate and result in a net gain of 2 ATP molecules.
done
clear
View Answer play_arrow
question_answer 151) One mole of ATP on hydrolysis yield energy equivalent to
A)
7k cal
done
clear
B)
12 k cal
done
clear
C)
24k cal
done
clear
D)
36 k cal.
done
clear
View Answer play_arrow
question_answer 152) The two main vectors used in genetic engineering to carry genes into bacteria are
A)
plasmids and bacteriophages
done
clear
B)
plastids and plasmids
done
clear
C)
plastids and bacteriophages
done
clear
D)
plasmids and phasmids.
done
clear
View Answer play_arrow
question_answer 153) If father has blood group A and mother has blood group\[O\]then which one of the following blood groups may be found in their son?
A)
B
done
clear
B)
AB
done
clear
C)
\[O\]
done
clear
D)
B, AB or\[O\].
done
clear
View Answer play_arrow
question_answer 154) Abnormal cell with random number of chromosomes is
A)
aneuploid
done
clear
B)
polyploid
done
clear
C)
heterokaryotic
done
clear
D)
haploid.
done
clear
View Answer play_arrow
question_answer 155) A drug called colchicine is shown to interfere specially with spindle microtubule formation during mitosis. This would result to
A)
dehydration of chromosomes
done
clear
B)
despiralization of chromosomes
done
clear
C)
arrest of chromosomes movements
done
clear
D)
arrest of centrioles movements.
done
clear
View Answer play_arrow
question_answer 156) Estimates place current fish production in India at about
A)
2.85 million metric tonnes
done
clear
B)
3.00 million metric tonnes
done
clear
C)
3.75 million metric tonnes
done
clear
D)
4.78 million metric tonnes.
done
clear
View Answer play_arrow
question_answer 157) Which is not true in muscle contraction?
A)
actin and myosin make actomyosin
done
clear
B)
phosphate reserve comes from CPK
done
clear
C)
chemical energy is converted into mechanical energy
done
clear
D)
mechanical energy is converted into chemical energy.
done
clear
View Answer play_arrow
question_answer 158) How many mitotic divisions are needed for a single cell to make 256 cells?
A)
8
done
clear
B)
16
done
clear
C)
32
done
clear
D)
64
done
clear
View Answer play_arrow
question_answer 159) X-chromosomes of female in a sex-linked inheritance case can be passed on to
A)
only female progeny
done
clear
B)
only male progeny
done
clear
C)
only in grand daughter
done
clear
D)
male and female progeny.
done
clear
View Answer play_arrow
question_answer 160) Water soluble droplets of fatty acids, phospholipids and bile salts are
A)
P-lipoprotein
done
clear
B)
chylomicrons
done
clear
C)
micelle
done
clear
D)
triglycerides.
done
clear
View Answer play_arrow
question_answer 161) What is the end product of glycolysis of a glucose molecule?
A)
fructose 1,6- diphosphate
done
clear
B)
pyruvate and ATP
done
clear
C)
phosphoglyceraldehyde
done
clear
D)
lactic acid and ATP.
done
clear
View Answer play_arrow
question_answer 162) Hybridoma is biotechnique which involves fusion of
A)
B-cell withT-cell
done
clear
B)
T-cell with spleen cell
done
clear
C)
spleen cell with myeloma cell
done
clear
D)
myeloma cell with B-cell.
done
clear
View Answer play_arrow
question_answer 163) Salivary gland is
A)
compound alveolar
done
clear
B)
compound tubular
done
clear
C)
alveolar
done
clear
D)
tubular.
done
clear
View Answer play_arrow
question_answer 164) In vertebrates the nerve growth factor occurs in the
A)
salivary secretions
done
clear
B)
gastric secretions
done
clear
C)
pancreatic secretion
done
clear
D)
lacrymal secretion.
done
clear
View Answer play_arrow
question_answer 165) The titin filaments bind the
A)
thin filaments to Z line
done
clear
B)
thick filaments to Z line
done
clear
C)
thin filaments to thick filaments
done
clear
D)
thick filaments to M line.
done
clear
View Answer play_arrow
question_answer 166) Serum differs from plasma in having
A)
excess of fibrinogen
done
clear
B)
absence of fibrinogen
done
clear
C)
excess of haemocyanin
done
clear
D)
absence of haemoglobin.
done
clear
View Answer play_arrow
question_answer 167) The spread of cancer cells from one part of the body to the another part is known as
A)
homeostasis
done
clear
B)
metastasis
done
clear
C)
metapophysis
done
clear
D)
metaplasia.
done
clear
View Answer play_arrow
question_answer 168) Sensitive technique used for the diagnosis of AIDS is
A)
ELISA
done
clear
B)
northern blot
done
clear
C)
southern blot
done
clear
D)
immunodiffusion.
done
clear
View Answer play_arrow
question_answer 169) Downs syndrome is basically a
A)
physiological abnormality
done
clear
B)
neurological abnormality
done
clear
C)
sex-linked abnormality
done
clear
D)
autosomal abnormality.
done
clear
View Answer play_arrow
question_answer 170) Enzyme-catalysed reaction can take place as physiological temperatures because they
A)
are proteins
done
clear
B)
have active sites
done
clear
C)
lower the activation energy
done
clear
D)
are heat labile.
done
clear
View Answer play_arrow
question_answer 171) Michaelis constant (km) of an enzyme is substrate concentration at which the reaction attains
A)
its maximum velocity
done
clear
B)
half its maximum velocity
done
clear
C)
double its maximum velocity
done
clear
D)
its normal velocity
done
clear
View Answer play_arrow
question_answer 172) During stress, besides epinephrine and norepinephrine which other hormones are also released?
A)
thyroxine, cortisol, aldosterone and vasopressin
done
clear
B)
cortisol, aldosterone, vasopressin and glucagons
done
clear
C)
cortisol, vasopressin, insulin and parathormone
done
clear
D)
parathormone, vasopressin, glucagon and thyroxine.
done
clear
View Answer play_arrow
question_answer 173) Nerve gas affect neuromuscular activity by
A)
blocking the acetylcholine receptor sites
done
clear
B)
inhibiting the release of acetylcholine
done
clear
C)
inhibiting acetylcholinesterase
done
clear
D)
enchancing the release of acetylcholine.
done
clear
View Answer play_arrow
question_answer 174) Formation of which of the following requires maximum ATP?
A)
urea
done
clear
B)
ammonia
done
clear
C)
uric acid
done
clear
D)
trimethylamine oxide.
done
clear
View Answer play_arrow
question_answer 175) As compared to a sphere of similar diameter, biconcave erythrocytes have a
A)
20-30% greater surface area to volume ratio
done
clear
B)
50-60% greater surface area to volume ratio
done
clear
C)
20-30% smaller surface area to volume ratio
done
clear
D)
50-60% smaller surface area to volume ratio.
done
clear
View Answer play_arrow
question_answer 176) Which of the following factors activates platelet adhesion to damaged blood vessel?
A)
Hagemanns factor
done
clear
B)
platelet factor IV
done
clear
C)
Von Willebrand factor
done
clear
D)
tissue factor.
done
clear
View Answer play_arrow
question_answer 177) The enzyme which acts on protein in alkaline medium is
A)
pepsin
done
clear
B)
renin
done
clear
C)
trypsin
done
clear
D)
all of these.
done
clear
View Answer play_arrow
question_answer 178) Absorption of toxic amounts of iron due to a genetically defective control mechanisms is known as
A)
haematochromatosis
done
clear
B)
haemosiderosis
done
clear
C)
haemoptysis
done
clear
D)
haematoma.
done
clear
View Answer play_arrow
question_answer 179) The cranial nerve which supplies internal viscera is
A)
trigeminal
done
clear
B)
trochlear
done
clear
C)
vagus
done
clear
D)
auditory.
done
clear
View Answer play_arrow
question_answer 180) Name the bacterium that helps in nitrogen fixation during anaerobic condition?
A)
Azotobacter
done
clear
B)
Pseudomonas
done
clear
C)
Clostridium
done
clear
D)
Nitrosomonas.
done
clear
View Answer play_arrow
question_answer 181) Retrogressive metamorphosis occurs in
A)
Amphioxus
done
clear
B)
Herdmania
done
clear
C)
Nereis
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 182) Respiration in Peripatus is by
A)
trachea
done
clear
B)
book lungs
done
clear
C)
general body surface
done
clear
D)
lungs.
done
clear
View Answer play_arrow
question_answer 183) Choose the odd statement for RNA.
A)
sugar moiety is ribose
done
clear
B)
uracil replaces thymine
done
clear
C)
single stranded
done
clear
D)
exclusive to viruses.
done
clear
View Answer play_arrow
question_answer 184) When purine substituted by pyrimidine, the resulting mutation is
A)
transition mutation
done
clear
B)
transversion mutation
done
clear
C)
point mutation
done
clear
D)
suppressive mutation
done
clear
View Answer play_arrow
question_answer 185) Inside a cell, the respiratory enzyme are contained in
A)
lysosomes
done
clear
B)
ribosomes
done
clear
C)
acrosomes
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 186) The protein synthesizing system is associated with
A)
ribosomes
done
clear
B)
microtubules
done
clear
C)
episomes
done
clear
D)
mitochondrial membrane.
done
clear
View Answer play_arrow
question_answer 187) Non-essential amino-acids combination is
A)
valine, leucine, glycine, alanine
done
clear
B)
glycine, serine, proline, glutamic acid
done
clear
C)
proline, aspartic acid, glutamic acid, methionine
done
clear
D)
cysteine, tyrosine, alanine, isoleucine.
done
clear
View Answer play_arrow
question_answer 188) The first self-duplicating units proposed have been synthesized in the primitive oceans are
A)
enzymes
done
clear
B)
genes
done
clear
C)
proteins
done
clear
D)
viruses.
done
clear
View Answer play_arrow
question_answer 189) Animal cell in which centrosome is not present is of
A)
Amoeba
done
clear
B)
Paramecium
done
clear
C)
Trypanosoma
done
clear
D)
Plasmodium.
done
clear
View Answer play_arrow
question_answer 190) The interphase nuclei of a person are showing two sex chromatins/nucleus. The possible genotype of the person is
A)
\[44+xxy\]
done
clear
B)
\[44+xyy\]
done
clear
C)
\[44+xxx\]
done
clear
D)
\[44+xxyy.\]
done
clear
View Answer play_arrow
question_answer 191) Which of the following viruses cause common cold?
A)
adenovirus
done
clear
B)
simian virus 40
done
clear
C)
T4 virus
done
clear
D)
MSZ virus.
done
clear
View Answer play_arrow
question_answer 192) The fate of a hormone bound to the specific receptors on the cell surface can be traced through
A)
X-ray
done
clear
B)
laser photo-bleaching
done
clear
C)
ultra-scanning
done
clear
D)
resonance imaging.
done
clear
View Answer play_arrow
question_answer 193) Technique to know the sex, congenital diseases and metabolic disorders is known as
A)
amniocentesis
done
clear
B)
castration
done
clear
C)
ovariectomy
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 194) Spiders belong to
A)
mandibulata
done
clear
B)
chelicerata
done
clear
C)
trilobata
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 195) The communities of primary succession are called as
A)
sub sere
done
clear
B)
meso sere
done
clear
C)
pioneer sere
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 196) Which is not a fossil?
A)
brachiopod
done
clear
B)
coprolite
done
clear
C)
Etirepterids
done
clear
D)
Balanoglossus.
done
clear
View Answer play_arrow
question_answer 197) The phenotypic variation is restricted to certain clear cut characteristics. This variation is commonly known as
A)
qualitative inheritance
done
clear
B)
quantitative inheritance
done
clear
C)
polygenic inheritance
done
clear
D)
complex inheritance.
done
clear
View Answer play_arrow
question_answer 198) The nature of photosynthesis in blue-green algae is
A)
oxygenic
done
clear
B)
anoxygenic
done
clear
C)
anaerobic
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 199) To control the stored grain pests the better and safe method is
A)
the use of chemical pesticides in small amounts
done
clear
B)
the use of less toxic pesticides
done
clear
C)
the biological control of pests
done
clear
D)
fumigation.
done
clear
View Answer play_arrow
question_answer 200) Movement of stomatal opening and closing and opening of Oxalis flower share the common feature that both are
A)
photonastic
done
clear
B)
thigmonastic
done
clear
C)
seismonastic
done
clear
D)
thermonastic.
done
clear
View Answer play_arrow
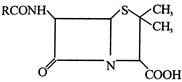 In ampicillin R =
In ampicillin R =
