question_answer 1) A body falls from rest under gravity. Its speed is v when it has lost an amount U of its potential energy. The mass of the body is
A)
\[\frac{Ug}{{{v}^{2}}}\]
done
clear
B)
\[\frac{{{v}^{2}}}{2g}\]
done
clear
C)
\[\frac{U}{g}\]
done
clear
D)
\[\frac{2U}{{{v}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 2)
Two blocks of masses\[{{m}_{1}}\]and\[{{m}_{2}}\]are lying on a horizontal frictionless table connected by a weightless string as shown in the diagram. The blocks are pulled by a horizontal force F applied to \[{{m}_{1}}\] The common acceleration of the blocks is
A)
\[F/({{m}_{1}}+{{m}_{2}})\]
done
clear
B)
\[F/{{m}_{1}}\]
done
clear
C)
\[F/{{m}_{2}}\]
done
clear
D)
\[(F+T)/{{m}_{2}}\]
done
clear
View Answer play_arrow
question_answer 3) If the net external force on a system of masses is zero. Which of the following is/are always conserved?
A)
total kinetic energy
done
clear
B)
total linear momentum
done
clear
C)
total angular momentum
done
clear
D)
total kinetic energy as well as linear momentum
done
clear
View Answer play_arrow
question_answer 4) A body is moving under the action of two forces\[{{\overrightarrow{F}}_{1}}=2\hat{i}-5\hat{j};{{\overrightarrow{F}}_{2}}=3\hat{i}-4\hat{j}\] Its velocity will become uniform under a third force\[{{\overrightarrow{F}}_{3}}\]given by
A)
\[5\hat{i}-\hat{j}\]
done
clear
B)
\[-5\hat{i}-\hat{j}\]
done
clear
C)
\[5\hat{i}+\hat{j}\]
done
clear
D)
\[-5\hat{i}+9\hat{j}\]
done
clear
View Answer play_arrow
question_answer 5) A cannon at the ground fires a shell at speed of 50 km/hr. If the angle of projection is\[30{}^\circ ,\] the range of the shell (take\[g=10\text{ }m/{{s}^{2}}\]) is, in km.
A)
\[2.5\sqrt{{}}3\]
done
clear
B)
\[62.5\sqrt{{}}3\]
done
clear
C)
\[125\sqrt{{}}3\]
done
clear
D)
\[9.65\sqrt{{}}3\times {{10}^{-3}}\]
done
clear
View Answer play_arrow
question_answer 6) A body starts moving with a velocity of 6 m/s making an angle of\[30{}^\circ \]with the x-axis. It has a uniform acceleration of\[3\text{ }m/{{s}^{2}}\]in the direction of\[x-\]axis. After 5 seconds, the ^-component of its velocity will be, in m/s.
A)
\[3\sqrt{{}}3\]
done
clear
B)
15
done
clear
C)
18
done
clear
D)
21
done
clear
View Answer play_arrow
question_answer 7) Two vectors \[\overrightarrow{A}=4\hat{i}+5\hat{j}+r\hat{k}\] and\[\overrightarrow{B}=2\hat{i}-4\hat{j}+6\hat{k}\] are perpendicular to each other if the scalar r is
A)
4
done
clear
B)
2
done
clear
C)
0
done
clear
D)
\[-2\]
done
clear
View Answer play_arrow
question_answer 8) A car is moving due north accelerated in the same direction, while a train is moving parallel to it also due north with a uniform speed of 100 km/hr. At the time when the speed of the car is 80 km/hr, an observer in the train finds the car speeding
A)
due south at 20 km per hour, accelerated due north
done
clear
B)
due north at 20 km per hour, accelerated due south
done
clear
C)
due south at 180 km per hour, accelerated due north
done
clear
D)
due north at 180 km per hour, accelerated due south
done
clear
View Answer play_arrow
question_answer 9) An\[\alpha -\]particle enters a straight hollow tube 1 m long with initial speed of\[1.0\times {{10}^{3}}\] m/s. After travelling horizontally with uniform acceleration. It leaves the tube with the speed of\[3.0\times {{10}^{3}}m/s\]. The time spent by the particle inside the tube is, in microseconds.
A)
1000
done
clear
B)
500
done
clear
C)
250
done
clear
D)
50
done
clear
View Answer play_arrow
question_answer 10) The dimensional formula for latent heat (in energy units) is
A)
\[M{{L}^{2}}{{T}^{-1}}\]
done
clear
B)
\[ML{{T}^{-2}}\]
done
clear
C)
\[{{M}^{0}}{{L}^{2}}{{T}^{-2}}\]
done
clear
D)
\[M{{L}^{2}}{{T}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 11) Average kinetic energy of an ideal gas consisting of N molecules is 3/2 NkT, where k is
A)
gas constant
done
clear
B)
Stefan constant
done
clear
C)
Boltzmann constant
done
clear
D)
Planck constant
done
clear
View Answer play_arrow
question_answer 12) Youngs modulus for the material of a wire depends On
A)
stretching force
done
clear
B)
length of the wire
done
clear
C)
cross-section area
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 13) When a body floats in water\[{{\left( \frac{1}{3} \right)}^{rd}}\]of its volume remains outside, whereas\[{{\left( \frac{3}{4} \right)}^{th}}\]of its volume remains outside when it floats in a liquid. The density of the liquid, in gm/cc, is
A)
8/3
done
clear
B)
4/3
done
clear
C)
9/4
done
clear
D)
3/2
done
clear
View Answer play_arrow
question_answer 14) A liquid drop of radius R breaks into 27 small drops. What will be the change in surface energy if the surface tension of the liquid is\[T\]?
A)
\[8\pi {{R}^{2}}T\]
done
clear
B)
\[12\pi {{R}^{2}}T\]
done
clear
C)
\[8{{\pi }^{2}}{{R}^{2}}T\]
done
clear
D)
\[12{{\pi }^{2}}{{R}^{2}}T\]
done
clear
View Answer play_arrow
question_answer 15) A stone is dropped from the top of a tower of height H. At the same time a second stone is projected vertically with a velocity\[\sqrt{3gH}\]from the foot of the tower. At what height, from the foot of the tower, will the two meet?
A)
\[3H/4\]
done
clear
B)
\[5H/6\]
done
clear
C)
\[3H/8\]
done
clear
D)
\[H/2\]
done
clear
View Answer play_arrow
question_answer 16) A body of mass m is projected to describe a parabolic path in vertical plane with velocity \[(3\hat{i}+8\hat{j})\]m/s. The time of flight of the body is about
A)
3.2 sec
done
clear
B)
1.6 sec
done
clear
C)
6.4 sec
done
clear
D)
1.2 sec
done
clear
View Answer play_arrow
question_answer 17) Which of the following statements is correct?
A)
torque does not change the angular momentum
done
clear
B)
in a rotational motion, force along a radial vector changes the angular momentum
done
clear
C)
angular velocity, angular momentum and torque are axial vectors
done
clear
D)
angular momentum and torque are always perpendicular to each other
done
clear
View Answer play_arrow
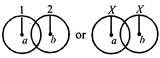
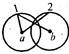
question_answer 18)
Three identical spheres of radius r and mass m touch each other (as shown). If the centre of mass of one of them is (0,0) then the centre of mass of the system is
A)
\[r,r/2\]
done
clear
B)
\[r,r/3\]
done
clear
C)
\[\frac{\sqrt{{}}3}{2}r,r\]
done
clear
D)
\[r,r\sqrt{{}}3\]
done
clear
View Answer play_arrow
question_answer 19) Work done by a force of 2 N acting along the \[x-\]axis on a body that moves from a point (3, 4) to another point (6, 8), distances being in metres, is
A)
6 J
done
clear
B)
8 J
done
clear
C)
10 J
done
clear
D)
16 J
done
clear
View Answer play_arrow
question_answer 20) A body of mass 100 g stops after travelling a certain distance on a rough horizontal surface. If its initial speed was 200 cm/s, the work done by it against the force of friction is
A)
\[-0.2\text{ }J\]
done
clear
B)
\[+0.2\text{ }J\]
done
clear
C)
cannot be calculated because the coefficient of friction is not given
done
clear
D)
cannot be calculated because the distance moved is not given
done
clear
View Answer play_arrow
question_answer 21) Two concentric spherical shells, made of copper, have radii r and\[R(R>r)\]and carry changes q and Q respectively. The potential at the surface of the inner spherical shell will be zero if Q is equal to
A)
\[-\frac{(r+R)}{r}q\]
done
clear
B)
\[\frac{(r-R)}{r}q\]
done
clear
C)
\[-\frac{R}{r}q\]
done
clear
D)
\[\frac{r}{R}q\]
done
clear
View Answer play_arrow
question_answer 22) An electric dipole of moment p is placed in a uniform electric field E. The couple required to rotate it through an angle 9 from the initial position is
A)
\[pE\tan \theta \]
done
clear
B)
\[pE\cos \theta \]
done
clear
C)
\[-pE\cos \theta \]
done
clear
D)
\[pE\sin \theta \]
done
clear
View Answer play_arrow
question_answer 23) The equation of a wave travelling on a string is \[y=5\cos \pi (0.5x-200t)cm\] Which is the wrong statement for the given wave?
A)
the velocity of the wave is 4 m/s
done
clear
B)
the two consecutive points on the wave having equal and opposite displacement are separated by a distance 2 cm.
done
clear
C)
the phase difference between two points separated by a distance of 0.42 m is\[\pi /2\]
done
clear
D)
the displacement of the point distant 200 cm from the source will be zero at \[t=\frac{201}{400}\]sec.
done
clear
View Answer play_arrow
question_answer 24) In a tank full of water, waves travel a distance of 45 cm in 3 s. If the distance between two consecutive crests is 3 cm, the frequency of the vibrator causing the waves, measured in Hz is
A)
5
done
clear
B)
7.5
done
clear
C)
3
done
clear
D)
15
done
clear
View Answer play_arrow
question_answer 25) A particle of mass m is executing damped oscillations. The force acting on the particle is given by the equation
A)
\[F=-k{{x}^{2}}-kv(t)\]
done
clear
B)
\[F=-kx-k{{d}^{2}}x/d{{t}^{2}}\]
done
clear
C)
\[F=-k{{x}^{2}}-kdx/dt\]
done
clear
D)
\[F=-kx-kdx/dt\]
done
clear
View Answer play_arrow
question_answer 26) If a spring of force constant\[k\]is divided into n equal parts and one such part is attached to a mass m then the period is given by
A)
\[T=2\pi \sqrt{m/k}\]
done
clear
B)
\[T=2\pi \sqrt{nm/k}\]
done
clear
C)
\[T=2\pi n\sqrt{m/k}\]
done
clear
D)
\[T=2\pi \sqrt{m/nk}\]
done
clear
View Answer play_arrow
question_answer 27) Physical significance of the first law of thermodynamics is that in a process involving heat.... remains constant.
A)
total energy
done
clear
B)
kinetic energy of the molecules
done
clear
C)
internal energy
done
clear
D)
temperature
done
clear
View Answer play_arrow
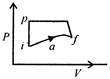
question_answer 28)
A system in going from\[i\]to\[f\]via a, as shown in the figure, does work of 30 cal and absorbs 40 cal heat. In going from\[f\]to\[i\]along the curved path, work done is\[-5\text{ }cal\]and the heat absorbed in cal, is
A)
\[-15\]
done
clear
B)
\[+15\]
done
clear
C)
\[+5\]
done
clear
D)
\[-5\]
done
clear
View Answer play_arrow
question_answer 29) An irreversible engine works between\[500{}^\circ K\] and\[200{}^\circ K\]. Its efficiency is
A)
0.6
done
clear
B)
0.7
done
clear
C)
0.8
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 30) Water boils at\[100{}^\circ C\]and freezes at\[0{}^\circ C\]at sea level atmospheric pressure. At higher pressure, water will boil at a
A)
lower temperature and ice melts at a lower temperature
done
clear
B)
lower temperature and ice melts at a higher temperature
done
clear
C)
higher temperature and ice melts at a higher temperature
done
clear
D)
higher temperature and ice melts at a lower temperature
done
clear
View Answer play_arrow
question_answer 31) In a series LCR circuit the resonance frequency is
A)
\[2\pi \sqrt{LC}\]
done
clear
B)
\[\sqrt{LC}\]
done
clear
C)
\[\frac{1}{2\pi \sqrt{LC}}\]
done
clear
D)
\[\frac{2}{\sqrt{LC}}\]
done
clear
View Answer play_arrow
question_answer 32) A magnet is allowed to fall through a metal ring. Its acceleration a during the fall is
A)
\[a<g\]
done
clear
B)
\[a>g\]
done
clear
C)
\[a=g\]
done
clear
D)
\[a=2g\]
done
clear
View Answer play_arrow
question_answer 33) A magnet 20 cm long and having a pole strength of 5 units is deflected through\[30{}^\circ \]from the magnetic meridian. If the earths magnetic field is 0.32 oersted, value of the deflecting couple will be, in dynes cm.
A)
16
done
clear
B)
8
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 34) A charge particle experiences maximum force in a magnetic field when it is
A)
at rest
done
clear
B)
moving in the direction of the field
done
clear
C)
moving in a direction making an angle of 45 to the field
done
clear
D)
moving perpendicular to the direction of the field
done
clear
View Answer play_arrow
question_answer 35) The weight of a horizontal current carrying wire can be supported by a magnetic field which is
A)
horizontal and perpendicular to the wire
done
clear
B)
horizontal and parallel to the wire
done
clear
C)
vertical upward
done
clear
D)
vertical downward
done
clear
View Answer play_arrow
question_answer 36) In a thermocouple, the neutral temperature is\[270{}^\circ C\]What will be the temperature of inversion if the temperature of the cold junction is\[20{}^\circ C\]?
A)
\[560{}^\circ C\]
done
clear
B)
\[520{}^\circ C\]
done
clear
C)
\[540{}^\circ C\]
done
clear
D)
\[500{}^\circ C\]
done
clear
View Answer play_arrow
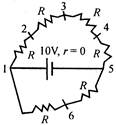
question_answer 37)
In the adjoining circuit, potential difference between points 2 and 5, in volts, is
A)
2.5
done
clear
B)
5.0
done
clear
C)
7.5
done
clear
D)
10.0
done
clear
View Answer play_arrow
question_answer 38) A high voltage electrical cable consists of a steel core surrounded by six aluminium conductors. If the resistance of the steel core is\[{{R}_{s}}\]and that of the aluminuium conductors is \[{{R}_{a}},\]the resistance of the composite cable is
A)
\[{{R}_{s}}+6{{R}_{a}}\]
done
clear
B)
\[\frac{1}{{{R}_{s}}}+\frac{1}{6{{R}_{a}}}\]
done
clear
C)
\[\frac{{{R}_{s}}{{R}_{a}}}{{{R}_{s}}+6{{R}_{a}}}\]
done
clear
D)
\[\frac{{{R}_{s}}{{R}_{a}}}{6{{R}_{s}}+{{R}_{a}}}\]
done
clear
View Answer play_arrow
question_answer 39) Resistivity of a conductor increases with temperature because
A)
number of free electrons increases
done
clear
B)
number of free electrons decreases
done
clear
C)
mean free path of free electrons increases
done
clear
D)
mean free path of free electrons decreases
done
clear
View Answer play_arrow
question_answer 40) A capactior of\[3\mu F\]capacity can be formed by connecting three\[2\mu F\]capacitors as in
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 41) A zero magnitude star has luminosity \[{{I}_{0}}=2.52\times {{10}^{-8}}W{{m}^{-2}}\]. Luminosity of a\[-10\] magnitude star is
A)
\[2.52\times {{10}^{-4}}W{{m}^{-2}}\]
done
clear
B)
\[2.52\times {{10}^{-5}}W{{m}^{-2}}\]
done
clear
C)
\[2.52\times {{10}^{-6}}W{{m}^{-2}}\]
done
clear
D)
\[2.52\times {{10}^{-7}}W{{m}^{-2}}\]
done
clear
View Answer play_arrow
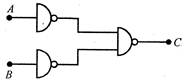
question_answer 42)
The following circuit is functionally equivalent to
A)
AND gate
done
clear
B)
NOR gate
done
clear
C)
NAND gate
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 43) If\[{{I}_{0}}\]and\[{{I}_{d}}\]denote drift and diffusion current respectively in a forward biased junction diode, then
A)
\[{{I}_{d}}<<{{I}_{0}}\]
done
clear
B)
\[{{I}_{d}}>>{{I}_{0}}\]
done
clear
C)
\[{{I}_{d}}={{I}_{0}}\]
done
clear
D)
\[{{I}_{0}}=0\]
done
clear
View Answer play_arrow
question_answer 44) A sample of radioactive substance has\[8\times {{10}^{8}}\]nuclei. Its half life is 20 minutes. The number of nuclei that will decay in one hour is
A)
\[2\times {{10}^{8}}\]
done
clear
B)
\[4\times {{10}^{8}}\]
done
clear
C)
\[1\times {{10}^{8}}\]
done
clear
D)
\[7\times {{10}^{8}}\]
done
clear
View Answer play_arrow
question_answer 45) In the hydrogen spectrum, spectral lines which are emitted due to the jump of electron from a higher orbit to the innermost orbit, correspond in frequency to the region.
A)
visible
done
clear
B)
ultraviolet
done
clear
C)
infrared
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 46) The de Broglie wavelength of a particle having mass m and velocity v is
A)
directly proportional to its mass
done
clear
B)
directly proportional to its energy
done
clear
C)
inversely proportional to its momentum
done
clear
D)
directly proportional to its momentum
done
clear
View Answer play_arrow
question_answer 47) The maximum frequency of continuous X-rays emitted from an X-ray tube operating at an accelerating voltage V depends
A)
nature of target material
done
clear
B)
nature of the gas in tube
done
clear
C)
current in the tube
done
clear
D)
accelerating voltage applied
done
clear
View Answer play_arrow
question_answer 48) If Youngs double slit experiment is performed using two identical conventional sources of light instead of two slit and one source then
A)
the interference fringes will be brighter
done
clear
B)
no fringes will be obtained
done
clear
C)
the interference fringes will be darker
done
clear
D)
the contrast between bright and dark fringes will increase
done
clear
View Answer play_arrow
question_answer 49) In a telescope, the distance between the objective and the eye piece is 24.0 cm. The focal length of the objective is 18.0 cm. Its magnifying power is
A)
3.0
done
clear
B)
3/2
done
clear
C)
2/3
done
clear
D)
0.3
done
clear
View Answer play_arrow
question_answer 50) The focal length of a convex lens made of glass\[{{(}^{a}}{{\mu }_{g}}=1.5)\]is 40 cm. When the lens is completely immersed in water\[{{(}^{a}}{{\mu }_{w}}=1.33)\]its focal length, in cm, will be about
A)
60
done
clear
B)
80
done
clear
C)
120
done
clear
D)
155
done
clear
View Answer play_arrow
question_answer 51)
Match List-I and List-II and select the correct answer using the code given below the lists List-I (outer electronic configuration) List-II (types of elements) A. \[[Ne]3{{s}^{1}}\] 1. alkali metal B. \[[Ne]3{{s}^{2}}3{{p}^{5}}3{{d}^{0}}4{{s}^{0}}\] 2. halogens(non-metallic) C. \[[Ar]3{{s}^{5}}4{{s}^{1}}\] 3. transition element D. \[[He]2{{s}^{2}}2{{p}^{6}}3{{s}^{0}}\] 4. inert (non-reactive) 5. inner transition 6. alkaline earth metals
A)
A-1, B-2, C-5, D-4
done
clear
B)
A-1, B-2, C-3, D-4
done
clear
C)
A-6, B-3, C-1, D-2
done
clear
D)
A-3, B-6, C-1, D-4
done
clear
View Answer play_arrow
question_answer 52) Lucas test is used to determine the type of
A)
alcohols
done
clear
B)
aldehydes
done
clear
C)
acids
done
clear
D)
phenols
done
clear
View Answer play_arrow
question_answer 53)
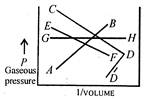
Which one of the following pressure versus volume plots represents Boyles Law? (see the accompanied diagram).
A)
line AB
done
clear
B)
line COD
done
clear
C)
line EF
done
clear
D)
line GH
done
clear
View Answer play_arrow
question_answer 54) Which of the following statements is incorrect in respect of ideal gaseous behaviour?
A)
all the molecules are identical and have intermolecular force of attraction
done
clear
B)
the gaseous molecules exchange energies during molecular collisions
done
clear
C)
the collisions are inelastic
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 55) Hofmann bromamide reaction is used to prepare
A)
primary amine
done
clear
B)
secondary amine
done
clear
C)
tertiary amine
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 56) Which one of the following is incorrect for the Bohr model of hydrogen atom?
A)
\[\frac{Z{{e}^{2}}}{{{r}^{2}}}=\frac{m{{v}^{2}}}{{{r}^{2}}}\]
done
clear
B)
angular momentum is quantized
done
clear
C)
mass of proton is ignored
done
clear
D)
none of the above
done
clear
View Answer play_arrow
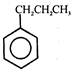
question_answer 57) The IUPAC name of \[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}} \end{smallmatrix}}{\mathop{CH-C{{H}_{2}}-C{{H}_{3}}}}\,\]
A)
4-Ethyl-2-methylhexane
done
clear
B)
2-Methyl-4-ethylhexane
done
clear
C)
3-lsobutylpentane
done
clear
D)
2-Ethyl-isopropylbutane
done
clear
View Answer play_arrow
question_answer 58) Heisenbergs uncertainty in position and momentum is shown by
A)
\[\Delta x.\Delta p\ge \frac{{{h}^{2}}}{4\pi }\]
done
clear
B)
\[\Delta x.\Delta p\ge \frac{h}{2\pi }\]
done
clear
C)
\[\Delta x.\Delta p\ge \frac{h}{4\pi }\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 59) Which one gives secondary alcohol when treated with Grignard Reagent?
A)
\[HCHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}CO\]
done
clear
D)
All the above
done
clear
View Answer play_arrow
question_answer 60) For isothermal change in the case of an ideal gas
A)
\[\Delta C=\Delta H-T\Delta S\]
done
clear
B)
\[\Delta C=-T\Delta S\]
done
clear
C)
\[\Delta A\text{ }=\Delta E-T\Delta S\]
done
clear
D)
None of the above
done
clear
View Answer play_arrow
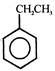
question_answer 61) Which of the following compound gives formic acid on hydrolysis?
A)
\[C{{H}_{3}}Cl\]
done
clear
B)
\[C{{H}_{2}}Cl\]
done
clear
C)
\[CHC{{l}_{3}}\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}Cl\]
done
clear
View Answer play_arrow
question_answer 62) The electropositive character decreases from
A)
Li to Cs (lithium to cesium) in the 1 group
done
clear
B)
\[Cl\]to\[I\](chlorine to iodine) in the VII group
done
clear
C)
\[Al\]to Na (aluminium to sodium) in the same period
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 63) Intramolecular hydrogen bonding is found in
A)
p-niiro phenol
done
clear
B)
o-nitro phenol
done
clear
C)
w-nitro phenol
done
clear
D)
phenol
done
clear
View Answer play_arrow
question_answer 64) Assertion: The rate of a reaction is enhanced in the presence of a positive catalyst. Reason: The positive catalyst provides an alternative path of higher activation energy
A)
both assertion and reason are true, and reason is the correct explanation of assertion
done
clear
B)
both assertion and reason are true but reason is NOT the correct explanation of assertion
done
clear
C)
asserton is true but reason is false
done
clear
D)
both assertion and reason are false
done
clear
View Answer play_arrow
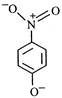
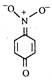
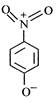
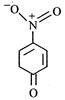
question_answer 65) The most unlikely representation of resonance structure of\[p-\]nitrophenoxide ion is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 66) The solubility product of\[AgCl\]is\[4.0\times {{10}^{-10}}\]at 298 K. The solubility of\[AgCl\]in\[0.04M\text{ }CaC{{l}_{2}}\] will be
A)
\[2.0\times {{10}^{-5}}M\]
done
clear
B)
\[1.0\times {{10}^{-4}}M\]
done
clear
C)
\[5.0\times {{10}^{-9}}M\]
done
clear
D)
\[2.2\times {{10}^{-4}}M\]
done
clear
View Answer play_arrow
question_answer 67) Which one of the following statements is not true?
A)
variable valencies are shown by the transition elements.
done
clear
B)
electrons enter into the\[3d-\]orbitals in the first series of the transition elements.
done
clear
C)
electropositive character decreases in lanthanide elements because of lanthanide contraction.
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 68) Pernicious Anaemia is caused due to the deficiency of vitamin
A)
\[{{B}_{1}}\]
done
clear
B)
\[{{B}_{2}}\]
done
clear
C)
\[{{B}_{6}}\]
done
clear
D)
\[{{B}_{12}}\]
done
clear
View Answer play_arrow
question_answer 69) Assertion: Spin quantum number can have the value\[+{\scriptstyle{}^{1}/{}_{2}}\] or\[-{\scriptstyle{}^{1}/{}_{2}}\]. Reason: (+) sign here signifies the wave function.
A)
both assertion and reason are correct and reason is the correct explanation of assertion
done
clear
B)
both assertion and reason are correct and reason is the not correct explanation of assertion
done
clear
C)
assertion is true but reason is false
done
clear
D)
both assertion and reason are false
done
clear
View Answer play_arrow
question_answer 70) Which one will give positive iodoform test?
A)
ethanol
done
clear
B)
propanone
done
clear
C)
2-Propanol
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 71) In\[{{H}_{2}}\]molecule, each of the two electrons
A)
is attracted by its own nucleus in accordance with the valence bond treatment:
done
clear
B)
is attracted by both the nuclei in the light of the molecular orbital consideration:
done
clear
C)
is attracted by both the nuclei in MO consideration and also they experience repulsion among themselves besides nuclear repulsion.
done
clear
D)
behave as stated above (a to c) in accordance with the VB and MO treatments.
done
clear
View Answer play_arrow
question_answer 72) Which of the following statements is not correct for an electron that has the quantum number\[n=4\]and\[m=2\]?
A)
the electron may have the quantum number \[s=+\text{ }1/2\]
done
clear
B)
the electron may have the quantum number\[l=2\].
done
clear
C)
the electron may have the quantum numbers \[l=3\]
done
clear
D)
the electron may have the quantum numbers\[l=0,1,2\]
done
clear
View Answer play_arrow
question_answer 73) A bond order of value 1/2 is found in
A)
\[He_{2}^{+}\]
done
clear
B)
\[H_{2}^{+}\]
done
clear
C)
\[He_{2}^{+}\]and \[H_{2}^{+}\]
done
clear
D)
all the above
done
clear
View Answer play_arrow
question_answer 74) Which one of the following compounds on nitration easily produces nitro derivative?
A)
\[{{C}_{6}}{{H}_{6}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}N{{O}_{2}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}C{{H}_{3}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}OC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 75) The correct outer electronic configuration of chromium atom is
A)
\[[Ar]3{{d}^{5}}4{{s}^{1}}\]
done
clear
B)
\[[Ar]3{{d}^{4}}4{{s}^{2}}\]
done
clear
C)
\[[Ar]3{{d}^{6}}\]
done
clear
D)
\[[Ar]3{{d}^{0}}4{{s}^{2}}4{{p}^{4}}\]
done
clear
View Answer play_arrow
question_answer 76) The formation of the product is facilitated by increasing the pressure in
A)
\[{{N}_{2}}(g)+{{O}_{2}}(g)2NO(g)\]
done
clear
B)
\[2N{{H}_{3}}(g){{N}_{2}}(g)+3{{H}_{2}}(g)\]
done
clear
C)
\[2NO(g){{N}_{2}}(g)+{{O}_{2}}(g)\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 77) The de Broglie relation is obtained by equating
A)
\[E={\scriptstyle{}^{1}/{}_{2}}m{{c}^{2}}\]and \[E=h\upsilon \]
done
clear
B)
\[E=m{{c}^{2}}\]and \[E=h\upsilon \]
done
clear
C)
\[E={{p}^{2}}/2m\]and \[E=h\upsilon \]
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 78) X on oxidation with alkaline\[KMn{{O}_{4}}\]gives benzoic acid. The X may be
A)
done
clear
B)
done
clear
C)
done
clear
D)
all of the above
done
clear
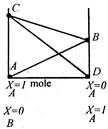
View Answer play_arrow
question_answer 79)
In the mixtures of two miscible volatile liquids obeying Raoults law, the correct behaviour is explained by
A)
AB stands for the vapour pressure of component B in presence of solute A
done
clear
B)
CD stands for the vapour pressure of solvent A in presence of solute B
done
clear
C)
BC stands for the total vapour pressure in accordance with the Daltons law of partial pressures
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 80) Octahedral, tetrahedral, and planar metal complexes have, respectively,
A)
\[{{d}^{2}},s{{p}^{3}},s{{p}^{3}},\]and\[s{{p}^{2}}\]hybridisation
done
clear
B)
\[s{{p}^{3}}{{d}^{2}},s{{p}^{3}},\]and\[sp\]hybridisation
done
clear
C)
\[{{d}^{2}}s{{p}^{3}}\]or\[s{{p}^{3}}{{d}^{2}},s{{p}^{3}},\]and\[ds{{p}^{2}}\]hybridization
done
clear
D)
\[{{d}^{2}}s{{p}^{3}},s{{p}^{3}},\]and\[s{{p}^{3}}{{d}^{2}}\]hybridization
done
clear
View Answer play_arrow
question_answer 81) The oxidation states of\[Mn\]in\[MnS{{O}_{4}},Mn{{O}_{2}}\] \[{{K}_{2}}Mn{{O}_{4}}\]and\[KMn{{O}_{4}}\]are respectively
A)
1, 2, 4, 4
done
clear
B)
2, 4, 6, 7
done
clear
C)
1, 2, 4, 6
done
clear
D)
2, 4, 7, 6
done
clear
View Answer play_arrow
question_answer 82) The\[_{92}{{U}^{238}}\]absorbs a thermal neutron. The correct sequence of radioactive decay, is [A]\[_{92}{{U}^{238}}{{+}_{0}}{{n}^{1}}{{\to }_{92}}{{U}^{239}}\xrightarrow[{{\frac{^{t}1}{2}}^{-23\,\min }}]{{}}93\,N{{p}^{239}}+{{\beta }^{-}}\] [B] \[_{93}N{{p}^{239}}{{\xrightarrow[{{\frac{^{t}1}{2}}^{-2-3day}}]{{}}}_{24}}P{{u}^{293}}+{{\beta }^{-}}\] [C] \[_{94}P{{u}^{239}}\xrightarrow[\begin{smallmatrix} by\,thermal\,neutron \\ \left( {{\frac{^{t}1}{2}}^{-24.000\,yrs}} \right) \end{smallmatrix}]{fissionable}{{\,}_{92}}{{U}^{235}}+\alpha \]is shown by
A)
A and B
done
clear
B)
B and C
done
clear
C)
C and A
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 83) Lowering of vapour, pressure,\[\Delta p\]: elevation in boiling point.\[\Delta {{T}_{b}}\]: and depression in freezing point,\[\Delta {{T}_{f}}\]of a solvent for the same molar concentration of each of the three solutes: [A] sugar, [B]\[NaCl,\] and [C]\[BaC{{l}_{2}}\]follow the sequence:
A)
\[\Delta p:A<B<C\]
done
clear
B)
\[\Delta {{T}_{b}}:C>B>A\]
done
clear
C)
\[\Delta {{T}_{f}}:A<B<C\]
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 84) In 0.1 M solutions of each of the following: Sucrose,\[NaCl,\]and\[MgC{{l}_{2}},\]the solutes will depress the freezing point of the solvent in the order:
A)
\[sucrose>NaCl>MgC{{l}_{2}}\]
done
clear
B)
\[NaCl>MgC{{l}_{2}}>sucrose\]
done
clear
C)
\[MgC{{l}_{2}}>sucrose>NaCl\]
done
clear
D)
\[MgC{{l}_{2}}>NaCl>sucrose\]
done
clear
View Answer play_arrow
question_answer 85) Which one among the following contains a phenolic\[-OH\]group
A)
citric acid
done
clear
B)
picric acid
done
clear
C)
formic acid
done
clear
D)
oxalic acid
done
clear
View Answer play_arrow
question_answer 86) The work done in expansion of an ideal gas from an initial volume\[{{V}_{1}}\]to a final\[{{V}_{2}}\]litres against a pressure of 1 atm at 300 K is
A)
\[2.303R\times 300\,log({{V}_{2}}/{{V}_{1}})\]
done
clear
B)
\[2.303R\times 300\,log({{V}_{1}}/{{V}_{2}})\]
done
clear
C)
\[1\text{ }atm\times ({{V}_{2}}/{{V}_{1}})\text{ }litre\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 87) For spontaneous reaction
A)
\[\Delta E\]or\[\Delta U\]must decrease
done
clear
B)
\[\Delta H\] must decrease
done
clear
C)
\[\Delta S\] must decrease
done
clear
D)
\[\Delta G\]must decrease
done
clear
View Answer play_arrow
question_answer 88) Which of the following depends on the path followed?
A)
internal energy change
done
clear
B)
enthalpy change
done
clear
C)
work done
done
clear
D)
free-energy change
done
clear
View Answer play_arrow
question_answer 89) The final product of the following sequence of reactions is \[C{{H}_{3}}Br+Mg\xrightarrow[{}]{Ether}A\xrightarrow[{}]{HCHO}B\xrightarrow[{}]{{{H}_{2}}O}C\]
A)
acetic acid
done
clear
B)
acetaldehyde
done
clear
C)
ethyl alcohol
done
clear
D)
formic acid
done
clear
View Answer play_arrow
question_answer 90) Caprolactum is the monomer of
A)
nylon-6
done
clear
B)
glyptal
done
clear
C)
dacron
done
clear
D)
melamine
done
clear
View Answer play_arrow
question_answer 91) Which one of the following statements is incorrect?
A)
ionisation potential decreases in a group while atomic volume increases
done
clear
B)
electron affinity increases from V to VII groups
done
clear
C)
transition metals give coloured ions and exhibit variable valencies
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 92) Acetic acid shows twice of its expected molecular weight value experimentally. This is because of
A)
its dissociation into\[{{H}^{+}}\]and\[C{{H}_{3}}CO{{O}^{-}}\] ions
done
clear
B)
colligative property measurement
done
clear
C)
molecular association through H-bonding
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 93) Which one of the following acids would you expect to be the strongest?
A)
\[FC{{H}_{2}}COOH\]
done
clear
B)
\[ClC{{H}_{2}}COOH\]
done
clear
C)
\[BrC{{H}_{2}}COOH\]
done
clear
D)
\[lC{{H}_{2}}COOH\]
done
clear
View Answer play_arrow
question_answer 94)
Match List 1 and List 2 and select the correct answer, using the codes given below the lists. Match: List 1 (compounds) List 2 (types of bond) A. \[MgO\] 1. Electrovalent B. \[{{H}_{2}}\] 2. Covalent C. \[Cu[N{{H}_{3}}]_{4}^{++}\] 3. Coordinate 4. Hydrogen bond
A)
A-1, B-2, C-3
done
clear
B)
A-1, B-4, C-3
done
clear
C)
A-2, B-4, C-1
done
clear
D)
A-3, B-2, C-1
done
clear
View Answer play_arrow
question_answer 95) The reducing reagent in Clemmensen reduction is
A)
\[LiAl{{H}_{4}}\]
done
clear
B)
\[Zn-NaOH\]
done
clear
C)
\[Na-NaOH\]
done
clear
D)
\[Zn-Hg-HCl\]
done
clear
View Answer play_arrow
question_answer 96) Assertion: According to Arrhenius equation \[K=A{{e}^{-{{E}_{a}}/RT}},\] ne energy of activation is independent of temperature. Reason: The plot of log k versus 1/T is linear
A)
assertion and reason both are correct and reason is the correct explanation of assertion
done
clear
B)
assertion and reason both are correct but reason is NOT the correct explanation of assertion
done
clear
C)
assertion is true but reason is false
done
clear
D)
both assertion and reason are false
done
clear
View Answer play_arrow
question_answer 97) Assertion: The reaction.\[A+B\](excess)\[\xrightarrow[{}]{k}C,\]for which\[\xrightarrow[dt]{d[C]}\propto [A],\]follows the first order kinetics. Reason: The order of a reaction is the number of molecules whose concentration determines the rate of reaction at a given temperature.
A)
both assertion and reason are true: and reason is the correct explanation of assertion
done
clear
B)
both assertion and reason are true: but reason is NOT the correct explanation of assertion
done
clear
C)
assertion is true but reason is false
done
clear
D)
assertion is false but reason is true
done
clear
View Answer play_arrow
question_answer 98) The amphoteric behaviour is shown by
A)
\[{{H}_{2}}C{{O}_{3}}\]and \[HCO_{3}^{-}\]
done
clear
B)
\[{{H}_{2}}C{{O}_{3}}\]and\[{{H}_{2}}O\]
done
clear
C)
\[HCO_{3}^{-}\] and \[{{H}_{3}}{{O}^{+}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 99) The incorrect match for the pH values and the corresponding concentration for the aqueous solutions of\[HCl,\text{ }NaCl,\]and\[NaOH\]is shown by
A)
\[\begin{align}
& \frac{HCl}{pHconcn(m/l)}\frac{NaCl}{pHconcn(m/l)}\frac{NaOH}{pHconcn(m/l)} \\
& \,\,\,\,\,\,\,\,\,3\times {{10}^{-3}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,7\times {{10}^{-5}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,11\times {{10}^{-3}} \\
\end{align}\]
done
clear
B)
\[\begin{align}
& \frac{HCl}{pHconcn(m/l)}\frac{NaCl}{pHconcn(m/l)}\frac{NaOH}{pHconcn(m/l)} \\
& \,\,\,\,\,\,\,\,\,\,4\times {{10}^{-4}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,5\times {{10}^{-5}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,10\times {{10}^{-4}} \\
\end{align}\]
done
clear
C)
\[\begin{align}
& \frac{HCl}{pHconcn(m/l)}\frac{NaCl}{pHconcn(m/l)}\frac{NaOH}{pHconcn(m/l)} \\
& \,\,\,\,\,\,\,\,\,\,5\times {{10}^{-5}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,7\times {{10}^{-7}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,9\times {{10}^{-5}} \\
\end{align}\]
done
clear
D)
\[\begin{align}
& \frac{HCl}{pHconcn(m/l)}\frac{NaCl}{pHconcn(m/l)}\frac{NaOH}{pHconcn(m/l)} \\
& \,\,\,\,\,\,\,\,\,6\times {{10}^{-6}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,7\times {{10}^{-6}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,8\times {{10}^{-6}} \\
\end{align}\]
done
clear
View Answer play_arrow
question_answer 100) Methyl bromide and ethyl bromide are mixed in equal proportion and the mixture is treated with sodium, the number of possible organic products is
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 101) The smallest known agents of the infection disease are
A)
viruses
done
clear
B)
mycoplasma
done
clear
C)
viroids
done
clear
D)
actinomycetes.
done
clear
View Answer play_arrow
question_answer 102) The best stage to produce haploid through anther culture is
A)
microspore mother cells
done
clear
B)
microspores
done
clear
C)
2- celled pollen grains
done
clear
D)
all of the above.
done
clear
View Answer play_arrow
question_answer 103) If a sporangium develops from a group of cell, the development of sporangium is known as
A)
heterosporangiate
done
clear
B)
homosporangiate
done
clear
C)
eusporangiate
done
clear
D)
leptosporangiate.
done
clear
View Answer play_arrow
question_answer 104) The first stable compound in Hatch Slack pathway is
A)
phosphoglyceric acid
done
clear
B)
lactic acid
done
clear
C)
oxalo acetic acid
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 105) Agar - agar which is extensively used in all microbiological studies and culture media is an important product of sea resources and is obtained from which group of algae?
A)
brown algae
done
clear
B)
green algae
done
clear
C)
red algae
done
clear
D)
diatoms & dinoflagellates.
done
clear
View Answer play_arrow
question_answer 106) Ribosome is made up of
A)
DNA\[+\]protein
done
clear
B)
RNA\[+\]protein
done
clear
C)
RNA\[+\]iipid
done
clear
D)
RNA\[+\]phosphate.
done
clear
View Answer play_arrow
question_answer 107) Which one of the following plant is most commonly used as green manure?
A)
Dalbergia sissoo
done
clear
B)
Polyaithea
done
clear
C)
Sesbania aculeate
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 108) If a fruit is developed from bicarpellary syncarpus inferior ovary with basal placement and with a single ovule, the fruit is termed as
A)
silique
done
clear
B)
cypsela
done
clear
C)
silicula
done
clear
D)
lomentun.
done
clear
View Answer play_arrow
question_answer 109) Which of the following statements is correct?
A)
during meiosis cytokinesis generally takes place
done
clear
B)
during meiosis cytokinesis may not take place
done
clear
C)
during meiosis cytokinesis takes place after first phase of meiosis and does not take place after second phase of meiosis
done
clear
D)
all of the above.
done
clear
View Answer play_arrow
question_answer 110) The first step in photosynthesis is
A)
formation of ATP
done
clear
B)
ionization of water
done
clear
C)
excitation of an electron of chlorophyll
done
clear
D)
attachment of\[C{{O}_{2}}\]to carbon sugar.
done
clear
View Answer play_arrow
question_answer 111) The ovule during its development undergoes \[180{}^\circ \]curvature bringing the micrbpyle and chalaza in a line. The development of ovule conforms to
A)
circinotropous
done
clear
B)
atropous
done
clear
C)
anatropous
done
clear
D)
amphitropous.
done
clear
View Answer play_arrow
question_answer 112) When the movement of plant or plant body is governed by the external stimulus it is known as
A)
paratonic movement
done
clear
B)
automonic movement
done
clear
C)
nastic movement
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 113) Senescence of plant organs is delayed by the application of
A)
auxin
done
clear
B)
gibberellin
done
clear
C)
cytokinin
done
clear
D)
ethylene.
done
clear
View Answer play_arrow
question_answer 114) During anther wall development if the middle layer is contributed by the inner secondary parietal layer, the development of anther wall layer conforms to
A)
dicotyledonous
done
clear
B)
monocotyledonous
done
clear
C)
basic type
done
clear
D)
reduced type.
done
clear
View Answer play_arrow
question_answer 115) Kranz type of leaf anatomy is found in
A)
\[{{C}_{1}}\] plants
done
clear
B)
\[{{C}_{4}}\]plants
done
clear
C)
\[{{C}_{3}}\]plants
done
clear
D)
\[{{C}_{2}}\]plants.
done
clear
View Answer play_arrow
question_answer 116) Reduction of NADP takes place in
A)
cyclic photophosphorylation
done
clear
B)
non - cyclic photophosphorylation
done
clear
C)
oxidative photophosphorylation
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 117) How many meiotic divisions would be required to produce 101 maize grains?
A)
101
done
clear
B)
26
done
clear
C)
127
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 118) Which one of the following decides cell to divide?
A)
\[{{G}_{1}}\]phase
done
clear
B)
\[{{G}_{2}}\]phase
done
clear
C)
S phase
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 119) The exact amount of energy available from each ATP (a part of the energy is lost as heat) is approximately
A)
7 K. calories
done
clear
B)
10 K. calories
done
clear
C)
5 K. calories
done
clear
D)
15 K. calories.
done
clear
View Answer play_arrow
question_answer 120) Which of the following is a source of variation?
A)
mutation
done
clear
B)
recombination
done
clear
C)
translocation
done
clear
D)
all of these.
done
clear
View Answer play_arrow
question_answer 121) Which one of the following occurs in radioactive fallout and behaves like calcium in biogeochemical cycling of material in ecosystem?
A)
strontium - 30
done
clear
B)
cobalt - 60
done
clear
C)
cesium-137
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 122) The formula for chlorophyll a is
A)
\[{{C}_{55}}{{H}_{70}}{{O}_{6}}{{N}_{4}}Mg\]
done
clear
B)
\[{{C}_{55}}{{H}_{72}}{{O}_{5}}{{N}_{4}}Mg\]
done
clear
C)
\[{{C}_{22}}{{H}_{72}}{{O}_{5}}{{N}_{4}}Mg\]
done
clear
D)
\[{{C}_{22}}{{H}_{72}}{{O}_{5}}{{N}_{4}}Mg\]
done
clear
View Answer play_arrow
question_answer 123) Degenerate codes are when
A)
the codons degenerate soon after the synthesis of polypeptide chain
done
clear
B)
one codon can code for more than one amino acids
done
clear
C)
a codon is non-functional and is also known as non-sense codon
done
clear
D)
the same amino acid can be coded by more than one codon.
done
clear
View Answer play_arrow
question_answer 124) Which one of the following element is responsible for Minamata diseases which is prevalent in industrial area due to pollution?
A)
cadmium
done
clear
B)
lead
done
clear
C)
mercury
done
clear
D)
zinc.
done
clear
View Answer play_arrow
question_answer 125) In our biosphere, the most important and vast area is covered by hydrosphere. Hydrosphere has
A)
1.4 billion cubic km of water
done
clear
B)
1.4 million cubic km of water
done
clear
C)
2.5 billion cubic km of water
done
clear
D)
1.5 billion cubic km of water.
done
clear
View Answer play_arrow
question_answer 126) The primary function of the light reaction of photosynthesis is
A)
production of ATP
done
clear
B)
production of \[ATP+NADP{{H}_{4}}\]
done
clear
C)
absorption of light
done
clear
D)
manufacture of food.
done
clear
View Answer play_arrow
question_answer 127) Myrmecophilly is a beneficial association between some flowering plants such as Cassia etc. and
A)
bacteria
done
clear
B)
mycoplasma
done
clear
C)
mycorrhiza
done
clear
D)
ants.
done
clear
View Answer play_arrow
question_answer 128) If the number of chromosomes in the pollen grains of Pinus is 12, what would be the number of chromosomes in its endosperm?
A)
12
done
clear
B)
36
done
clear
C)
6
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 129) A class of mutation induced by addition or deletion of a nucleotide is called
A)
missense
done
clear
B)
nonsense
done
clear
C)
substitution
done
clear
D)
frameshift.
done
clear
View Answer play_arrow
question_answer 130) If the cell ca of 2-celled proembryo divides transversely, the embryo development may conform to
A)
chenopodiad and asterad types
done
clear
B)
onagrad and solanad types
done
clear
C)
caryophyllad and onagrad types
done
clear
D)
solanad and chenopodiad types.
done
clear
View Answer play_arrow
question_answer 131) Monera includes
A)
actinomycetes only
done
clear
B)
cyanobacteria only
done
clear
C)
actinomycetes and cyanobacteria
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 132) Chipko Andolan (movement) which was started in 1970 in Garhwal Himalayas (Gopeshwar) near Alaknand river was for the first time started by
A)
Baba Amte
done
clear
B)
Maneka Gandhi
done
clear
C)
Chander Prasad Bhat
done
clear
D)
Sunder Lal Bahuguna.
done
clear
View Answer play_arrow
question_answer 133) In a vertical section of a dorsiventral leaf, phloem in the midrib vascular strand
A)
is not distinct
done
clear
B)
faces abaxial side
done
clear
C)
faces adaxial side
done
clear
D)
is surrounded by metaxylem
done
clear
View Answer play_arrow
question_answer 134) A natural phenomenon is commonly observed in lakes, ponds and ditches that there is a rich growth of algae and other micro-organisms which consume much of the dissolved oxygen, resulting the death of fish at night. The phenomenon is termed as
A)
litterization
done
clear
B)
transmigration
done
clear
C)
eutrophication
done
clear
D)
estuarization.
done
clear
View Answer play_arrow
question_answer 135) When a fungus completes its life cycle on two hosts, it is called
A)
heterothallic
done
clear
B)
monothallic
done
clear
C)
heteroecious
done
clear
D)
autoecious.
done
clear
View Answer play_arrow
question_answer 136) In order to reduce expenses over plantation for afforestation programme under the Social Forestry, a system has been developed which include forestry and agriculture. The system is named as
A)
selective harvest system
done
clear
B)
taungya system
done
clear
C)
jhum cultivation
done
clear
D)
block system.
done
clear
View Answer play_arrow
question_answer 137) The pyramid of biomass would be inverted in
A)
forest ecosystem
done
clear
B)
marine ecosystem
done
clear
C)
grass land ecosystem
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 138) When there is a bunch of flagella on one side, the bacteria are known as
A)
lophotrichous
done
clear
B)
amphitrichous
done
clear
C)
peritrichous
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 139) Reindeer Moss, a very important product in tundra biome and which constitutes the starting point to food chain in this region belongs to
A)
pteridophyta
done
clear
B)
bryophyta
done
clear
C)
lichens
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 140) The sequence and kinds of amino acids in a particular protein are determined by a sequence of
A)
two nucleotides
done
clear
B)
three nucleotides
done
clear
C)
four nucleotides
done
clear
D)
five nucleotides.
done
clear
View Answer play_arrow
question_answer 141) Subabul a fast growing tree and extensively used in social forestry programme to generate cheap fuel for rural population is botanically known as
A)
Acacia nilotica
done
clear
B)
Parkinsonia aculeata
done
clear
C)
Lezicaena leucocephala
done
clear
D)
Simmondsia chinensis.
done
clear
View Answer play_arrow
question_answer 142) Simmondsia chinensis is an oil yielding plant of Mexico and it has been recently introduced in Indian deserts. What is the common name of this plant?
A)
subabul
done
clear
B)
gauynie
done
clear
C)
winged bean
done
clear
D)
jojoba.
done
clear
View Answer play_arrow
question_answer 143) If the decomposers from an ecosystem go on strike from functioning as decomposers, the functioning of ecosystem will be adversely affective because
A)
energy flow in an ecosystem will be blocked
done
clear
B)
cycling of mineral and material will be stopped
done
clear
C)
both [a] and [b]
done
clear
D)
adversely affected for a short period and then function normally
done
clear
View Answer play_arrow
question_answer 144) Which one of the following plants is used in paint and varnish industry?
A)
Abies
done
clear
B)
Cedrus deodara
done
clear
C)
Pinus roxburghii
done
clear
D)
Pinus gerardiana.
done
clear
View Answer play_arrow
question_answer 145) Which one of the following is M.F.P?
A)
timber
done
clear
B)
resin and gums
done
clear
C)
plywood
done
clear
D)
lumber.
done
clear
View Answer play_arrow
question_answer 146) A typical foliage leaf comprises
A)
hypopodium
done
clear
B)
mesopodium
done
clear
C)
epipodium
done
clear
D)
all of these.
done
clear
View Answer play_arrow
question_answer 147) Intergeneric crosses are rarely successful through usual breeding techniques. Which of the following may be used to achieve success in this regard?
A)
embryo culture
done
clear
B)
hybridoma technique
done
clear
C)
somatic hybridization
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 148) The plant cells commonly without nucleus among the following are
A)
sieve tube elements
done
clear
B)
roots hairs
done
clear
C)
companion cells
done
clear
D)
albuminous cells.
done
clear
View Answer play_arrow
question_answer 149) Prokaryotic flagella are having
A)
unit membrane - enclosed fibre.
done
clear
B)
helically arranged protein molecule
done
clear
C)
microtubular\[9+2\]membrane enclosed structure
done
clear
D)
protein membrane enclosed fibre.
done
clear
View Answer play_arrow
question_answer 150) Goldfussia is an example of____where the leaves of different size are present in opposite phyllotaxy.
A)
heterophylly
done
clear
B)
anisophylly
done
clear
C)
opposite decussate
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 151) . The area cribrosa occurs at
A)
base of medullary pyramid
done
clear
B)
tip of medullary pyramid
done
clear
C)
the major calyx
done
clear
D)
the pelvis.
done
clear
View Answer play_arrow
question_answer 152) One major change accompanying the evolution of homo from australopithecus was
A)
bipedal locomotion
done
clear
B)
decrease in body size
done
clear
C)
increase in brain size
done
clear
D)
uniformity of teeth.
done
clear
View Answer play_arrow
question_answer 153) Coral reef formation is mainly related to
A)
sponges
done
clear
B)
anthozoans
done
clear
C)
molluscs
done
clear
D)
hydrozoans.
done
clear
View Answer play_arrow
question_answer 154) Under the wild life protection Act of the Government of India, the Indian elephant is in the category of
A)
protected species
done
clear
B)
endangered species
done
clear
C)
vulnerable species
done
clear
D)
rare species.
done
clear
View Answer play_arrow
question_answer 155) Edward Jenner was the first person who
A)
tried to develop vaccine
done
clear
B)
discovered penicillin
done
clear
C)
introduced the term cell
done
clear
D)
discovered the life of malaria in mosquito.
done
clear
View Answer play_arrow
question_answer 156) The organic compound having polyhydroxy aldehydes or ketones is
A)
carbohydrate
done
clear
B)
lipid
done
clear
C)
protein
done
clear
D)
vitamin.
done
clear
View Answer play_arrow
question_answer 157) Animal requiring minimum amount of water to produce urine are
A)
ureotelic
done
clear
B)
ammonotelic
done
clear
C)
uricotelic
done
clear
D)
chemotelic.
done
clear
View Answer play_arrow
question_answer 158) For X - linkage, males are called as
A)
heterozygous
done
clear
B)
hemizygous
done
clear
C)
autozygous
done
clear
D)
allozygous
done
clear
View Answer play_arrow
question_answer 159) Which is not related with eukaryotic\[mRNA\]?
A)
intron, exon
done
clear
B)
ribosomes binding site
done
clear
C)
poly A tail, Gppp Cap
done
clear
D)
okazaki piece.
done
clear
View Answer play_arrow
question_answer 160) The respiratory and cardiac centres are located in
A)
cerebrum
done
clear
B)
diencephalons
done
clear
C)
crura cerebri
done
clear
D)
medulla oblongata.
done
clear
View Answer play_arrow
question_answer 161) By weight the most abundant element in a cell is
A)
carbon
done
clear
B)
hydrogen
done
clear
C)
oxygen
done
clear
D)
sodium.
done
clear
View Answer play_arrow
question_answer 162) A child (foetus) may die if
A)
mother is\[R{{h}^{+}}\] and father \[R{{h}^{-}}\]
done
clear
B)
father is\[R{{h}^{+}}\]and mother\[R{{h}^{-}}\]
done
clear
C)
father is\[R{{h}^{+}}\]and mother\[R{{h}^{-}}\]
done
clear
D)
father is\[R{{h}^{+}}\]and so is mother.
done
clear
View Answer play_arrow
question_answer 163) All sensory information to be registered consciously by the forebrain must pass via the
A)
thalamus
done
clear
B)
reticular formation
done
clear
C)
cerebellum
done
clear
D)
pons.
done
clear
View Answer play_arrow
question_answer 164) A sex-linked disease which is lethal, not connected with blood and changes the sex ratio is
A)
colourblindness
done
clear
B)
muscular dystrophy
done
clear
C)
G6PD deficiency
done
clear
D)
haemophilia A and B.
done
clear
View Answer play_arrow
question_answer 165) Beer and buttermilk are the products of fermentation brought about by a microscopic organism called
A)
Saccharomyces cerevisae
done
clear
B)
Rhizopus stolonifer
done
clear
C)
Caedibacter taeniospiralis
done
clear
D)
Bacillus subtilis.
done
clear
View Answer play_arrow
question_answer 166) Which one of the following forms of RNAs is involved in receiving the message of genetic code from DNA for translation during protein synthesis?
A)
\[rRNA\]
done
clear
B)
\[mRNA\]
done
clear
C)
\[tRNA\]
done
clear
D)
all of these.
done
clear
View Answer play_arrow
question_answer 167) Which of the following groups contain both DNA and RNA?
A)
bacteria and higher organisms
done
clear
B)
tobacco mosaic virus and poliomyelitis virus
done
clear
C)
bacteriophages and adenovirus
done
clear
D)
human immuno deficiency virus and papilloma virus.
done
clear
View Answer play_arrow
question_answer 168) The stage of meiosis where in the centromere divides for the first and the only time, ensuring separation of daughter chromosomes, occur in
A)
anaphase II
done
clear
B)
telophase I
done
clear
C)
anaphase I
done
clear
D)
telophase II
done
clear
View Answer play_arrow
question_answer 169) In which substage ofprophase I, pairing between homologous chromosomes is completed throughout whole length and pairs are referred to as biyalents?
A)
diplotene
done
clear
B)
zygotene
done
clear
C)
leptotene
done
clear
D)
pachytene
done
clear
View Answer play_arrow
question_answer 170) The element particles on mitochondria are concerned with
A)
glycolysis
done
clear
B)
breakdown of ATP
done
clear
C)
Krebs cycle
done
clear
D)
oxidative phosphorylation.
done
clear
View Answer play_arrow
question_answer 171) Restriction enzymes recognize and cleave/cut at specific site of the molecules of
A)
DNA
done
clear
B)
carbohydrates
done
clear
C)
fats
done
clear
D)
vitamins.
done
clear
View Answer play_arrow
question_answer 172) A species with several subspecies is called a
A)
polytypic species
done
clear
B)
polytopic species
done
clear
C)
superspecies
done
clear
D)
monotypic species.
done
clear
View Answer play_arrow
question_answer 173) An enzyme which has multiple molecular forms in the same organism is termed as
A)
isozyme
done
clear
B)
isoenzyme
done
clear
C)
holoenzyme
done
clear
D)
apoenzyme.
done
clear
View Answer play_arrow
question_answer 174) Prokaryotes have
A)
large nuclei
done
clear
B)
single large nucleus
done
clear
C)
large and small nuclei
done
clear
D)
no defined nucleus.
done
clear
View Answer play_arrow
question_answer 175) The reaction between antibodies and soluble antigens is
A)
agglutination
done
clear
B)
neutralization
done
clear
C)
precipitation
done
clear
D)
lysis.
done
clear
View Answer play_arrow
question_answer 176) Release of neurotransmitter substance at the presynaptic membrane is medicated by
A)
Na ions
done
clear
B)
Ca ions
done
clear
C)
K ions
done
clear
D)
Mg ions.
done
clear
View Answer play_arrow
question_answer 177) Mammary gland is an example of a compound
A)
tubular gland
done
clear
B)
alveolar glands
done
clear
C)
tubulo - alveolar gland
done
clear
D)
saccular gland.
done
clear
View Answer play_arrow
question_answer 178) Transmission of signals or impulse through a nerve is
A)
physical phenomenon
done
clear
B)
chemical phenomenon
done
clear
C)
physico - chemical phenomenon
done
clear
D)
mechanical phenomenon.
done
clear
View Answer play_arrow
question_answer 179) Dopamine is a
A)
hormone
done
clear
B)
transmitter hormone
done
clear
C)
polysaccharide
done
clear
D)
enzyme.
done
clear
View Answer play_arrow
question_answer 180) Which one of the following will be untenable nucleotide combination?
A)
phosphate - deoxyribose - uracil
done
clear
B)
phosphate - ribose-uracil
done
clear
C)
phosphate - ribose-adenine .
done
clear
D)
phosphate - deoxyribose - cytosine.
done
clear
View Answer play_arrow
question_answer 181) The binomial system of nomenclature now followed universally, was first proposed by
A)
Aristotle
done
clear
B)
Caroleus Linnaeus
done
clear
C)
G. Simpson
done
clear
D)
E. Mayr.
done
clear
View Answer play_arrow
question_answer 182) Which of the following hormone is necessary for the development of secondary sexual characters, in mammals including human beings?
A)
progesterone
done
clear
B)
FSH
done
clear
C)
testosterone
done
clear
D)
estrogen.
done
clear
View Answer play_arrow
question_answer 183) Vitamin\[{{B}_{12}}\]is also known as
A)
riboflavin
done
clear
B)
pyridoxine
done
clear
C)
folic acid
done
clear
D)
cdbalamine.
done
clear
View Answer play_arrow
question_answer 184) The disorders of AIDS is characterized by
A)
decrease in the number of killer T-cells
done
clear
B)
decrease in the number of suppressor T-cells
done
clear
C)
decrease in the number of helper T-cells
done
clear
D)
increase in the number of helper T-cells.
done
clear
View Answer play_arrow
question_answer 185) Beri beri is caused by the deficiency of
A)
thiamine
done
clear
B)
niacin
done
clear
C)
folic acid
done
clear
D)
biotin.
done
clear
View Answer play_arrow
question_answer 186) Sucrase hydrolysis sucrose into
A)
glucose and galactose
done
clear
B)
glucose and fructose
done
clear
C)
galactose and fructose
done
clear
D)
glucose and ribose.
done
clear
View Answer play_arrow
question_answer 187) Which one of the following is not a protein digesting enzyme?
A)
pepsin
done
clear
B)
ptyalin
done
clear
C)
trypsin
done
clear
D)
chymotrypsin.
done
clear
View Answer play_arrow
question_answer 188) One method of biotechnology for Animal Breeding Programmes to improve yield of milk/meat/wool in catties, goat, sheep, is to incorporate new/foreign genes into the genome of their fertilized eggs to produce
A)
hybrids
done
clear
B)
\[{{F}_{1}}\] generation
done
clear
C)
transgenic individuals
done
clear
D)
\[{{F}_{2}}\] generation.
done
clear
View Answer play_arrow
question_answer 189) Plasmid found in bacteria are used as vectors in molecular biology/biotechnology work. These genetic elements of bacteria are
A)
chromosomal
done
clear
B)
extra-chromosomal
done
clear
C)
mitochondrial
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 190) Germplasm preservation at ultra-low temperatures around\[-196{}^\circ C,\] is called as
A)
ultra - preservation
done
clear
B)
cryo - preservation
done
clear
C)
chemical preservation
done
clear
D)
physical preservation.
done
clear
View Answer play_arrow
question_answer 191) Scorpions belong to the phylum
A)
annelida
done
clear
B)
echinodermata
done
clear
C)
arthropoda
done
clear
D)
porifera.
done
clear
View Answer play_arrow
question_answer 192) Ommatidia are the units that constitute the compound eyes in
A)
fish
done
clear
B)
insects
done
clear
C)
mammals
done
clear
D)
birds.
done
clear
View Answer play_arrow
question_answer 193) Annelids like the earthworms, Pheretima posthuma, excrete urine that is
A)
isotonic
done
clear
B)
hypertonic
done
clear
C)
hypotonic
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 194) Animals that tolerate a very narrow range of temperature fluctuation are called as
A)
eurythermal
done
clear
B)
stenothermal
done
clear
C)
euryhaline
done
clear
D)
stenohaline.
done
clear
View Answer play_arrow
question_answer 195) Which is related to Darwin?
A)
Galapagos island
done
clear
B)
Andaman islands
done
clear
C)
Hawiian islands
done
clear
D)
Mauritius.
done
clear
View Answer play_arrow
question_answer 196) Competition between individuals of the same species, is called
A)
inter - specific competition
done
clear
B)
intra - specific competition
done
clear
C)
predation
done
clear
D)
population interaction.
done
clear
View Answer play_arrow
question_answer 197) Population exploiting a resource rapidly, and showing unbounding increase in numbers are
A)
\[k-\]selected
done
clear
B)
\[r-\]selected
done
clear
C)
growth selected
done
clear
D)
unselected.
done
clear
View Answer play_arrow
question_answer 198) The essential components of ecosystem, without which ecosystems cannot function, are
A)
consumer organisms
done
clear
B)
secondary consumer organisms
done
clear
C)
sunlight, green plants
done
clear
D)
decomposer organisms.
done
clear
View Answer play_arrow
question_answer 199) Biosphere consists of basic units known as
A)
biomes
done
clear
B)
regional ecosystem patterns
done
clear
C)
ecosystems
done
clear
D)
geographical patterns.
done
clear
View Answer play_arrow
question_answer 200) Closely related species living in the same area/ habitat, are called as
A)
allopatric
done
clear
B)
sympatric
done
clear
C)
allochoronic
done
clear
D)
siblings.
done
clear
View Answer play_arrow