Some Important Polymer and Their Uses
Category : JEE Main & Advanced
Rubber
| Rubber | Monomers | Formula | Applications |
| (i) Neoprene rubber | \[\underset{\text{Chloroprene}}{\mathop{C{{H}_{2}}=\underset{Cl\,}{\mathop{\underset{|}{\mathop{C}}\,-}}\,CH=C{{H}_{2}}}}\,\] | \[{{\left( -C{{H}_{2}}-\underset{Cl\,}{\mathop{\underset{|}{\mathop{C}}\,=}}\,CH-C{{H}_{2}}- \right)}_{n}}\] | Making automobile, refrigerator parts and electric wire. |
| (ii) Styrene Butadiene Rubber (SBR) or Buna-S | 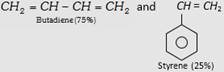 |
 |
Making of tyre and other mechanical rubber goods. |
| (iii) Butyl rubber | 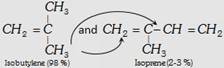 |
\[{{\left( -C{{H}_{2}}-\overset{C{{H}_{3}}\,\,\,\,\,\,}{\mathop{\overset{|}{\mathop{C}}\,=CH}}\,-C{{H}_{2}}-\underset{C{{H}_{3}}\,\,\,\,\,\,\,}{\overset{C{{H}_{3}}\,\,\,\,\,\,\,}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,-C{{H}_{2}}}}}\,- \right)}_{n}}\] | Making of toys, tyre, tube etc. |
| (iv) Nitrile rubber or Buna N or GRA | \[\underset{\text{Butadiene }(75%)}{\mathop{C{{H}_{2}}=CH-CH=C{{H}_{2}}}}\,\]and \[\underset{\text{Acrylonitrile }(25%)}{\mathop{C{{H}_{2}}=CH-CN}}\,\] | \[{{\left( -C{{H}_{2}}-\underset{CN}{\mathop{\underset{|}{\mathop{C}}\,H}}\,-C{{H}_{2}}-CH=CH-C{{H}_{2}}- \right)}_{n}}\] | Used for make of fuel tank. |
| (v) Polysulphide rubber (Thiokol) | \[\underset{\text{Ethylene dichloride}}{\mathop{Cl-C{{H}_{2}}-C{{H}_{2}}-Cl}}\,\] and \[\underset{\text{Sodium tetrasulphide}}{\mathop{N{{a}_{2}}{{S}_{4}}}}\,\] | \[{{(-C{{H}_{2}}-C{{H}_{2}}-S-S-S-S-)}_{n}}\] | Used in the manufacture of hoses and tank lining, engine gasket and rocket fuel. |
| (vi) Silicone rubber | \[\underset{\text{Chlorosilanes}}{\mathop{Cl-\overset{C{{H}_{3}}\,\,\,\,\,\,\,\,}{\mathop{\underset{Cl\,\,}{\mathop{\underset{|}{\overset{|}{\mathop{S}}}\,i-}}\,C{{H}_{3}}}}\,}}\,\] | \[{{\left( -O-\underset{C{{H}_{3}}}{\overset{C{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{S}}}\,i-\,}}}\, \right)}_{n}}\] | Silicon rubber |
| (vii) Polyurethane rubber | \[\underset{\text{Ethylene glycol}}{\mathop{HOC{{H}_{2}}-C{{H}_{2}}OH}}\,\] and \[\underset{\text{Ethylene di-isocyanate}}{\mathop{\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,=N-CH=CH-N=C=O}}\,\] | In the manufacture of fibre. Paints and heat insulator. |
Plastics and resin
| Name of polymer | Abbreviat-ion | Starting materials (monomers) | Nature of polymer | Properties | Applications |
| (i) Polyolefines | |||||
| (a) Polyethylene or polyethene | LDPE (Low density polyethene) | \[C{{H}_{2}}=C{{H}_{2}}\] | Low density homopolymer (branched) chain growth. | Transparent, moderate tensile strength, high toughness. | Packing material carry bags, insulation for electrical wires and cables. |
| HDPE (high density polyethene) | \[C{{H}_{2}}=C{{H}_{2}}\] | High density homopolymer (linear) chain growth. | Transluscent, chemically inert, greater tensile strength, toughness. | Manufacture of buckets, tubs, house ware, pipes, bottles and toys. | |
| (b) Polypropylene or polypropene | PP | \[C{{H}_{3}}CH=C{{H}_{2}}\] | Homopolymer, linear, chain growth. | Harder and stronger than polyethene. | Packing of textiles and foods, liners for bags, heat shrinkage wraps, carpet fibres, ropes, automobile mouldings, stronger pipes and bottles. |
| (c) Polystyrene or Styron or styrofoam | \[{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\] | Homopolymer, linear, chain growth | Transparent | Plastic toys, house hold wares, radio and television bodies, refrigerator linings. | |
| (ii) Polyhaloolefines | |||||
| (a) Polyvinyl chloride | PVC | \[\underset{\text{Vinyl chloride}}{\mathop{C{{H}_{2}}=CH-Cl}}\,\] | Homopolymer chains growth | Thermoplastic | (i) Plasticised with high boiling esters PVC used in rain coats, hand bags, shower curtains, fabrics, shoe soles, vinyl flooring (ii) Good electrical insulator (iii) Hose pipes. |
| (b) Polytetrafluoroet-hylene or Teflon | PTFE | \[{{F}_{2}}C=C{{F}_{2}}\] | Homopolymer, high melting point | Flexible and inert to solvents boiling acids even aqua regia. Stable upto 598 K. | (i) For nonstick utensiles coating (ii) Making gaskets, pump packings valves, seals, non lubricated bearings. |
| (c) Polymonochlorotri-fluroroethylene | PCTFE | \[ClFC=C{{F}_{2}}\] | Homopolymer | Less resistant to heat and chemicals due to presence of chlorine atoms. | Similar to those of teflon. |
| (iii) Formaldehyde resins | |||||
| (a) Phenol formaldehyde resin or Bakelite | Phenol and formaldehyde | Copolymer, step growth | Thermosetting polymer, hard and brittle | (i) With low degree polymerisation as bindings glue for wood varnishes, lacquers. (ii) With high degree polymerisation for combs, for mica table tops, fountain pen barrels electrical goods (switches and plugs). | |
| (b) Melamine formaldehyde resin | Melamine and formaldehyde | Copolymer, step growth | Thermosetting polymer, hard but not so breakable. | Non-breakable crockery. | |
| (iv) Polyacrylates | |||||
| (a) Polymethacrylate (lucite, acrylite and plexiglass and perspex) | PMMA | \[C{{H}_{2}}=\overset{C{{H}_{3}}\,}{\mathop{\overset{|}{\mathop{C}}\,-C}}\,OOC{{H}_{3}}\] | Copolymer | Hard transparent, excellent light transmission, optical clarity better than glass takes up colours. | Lenses light covers lights, shades signboards transparent domes skylight aircraft window, dentures and plastic jewellery. |
| (b) Polyethylacrylate | \[C{{H}_{2}}=CH-COO{{C}_{2}}{{H}_{5}}\] | Copolymer | Tough, rubber like product |
Fibre
| Name of polymer | Abbreviation | Starting materials | Nature of polymer | Properties | Applications |
| (i) Polysters (a) Terylene or Dacron or mylar | PET (Polyethylene terephthalate) | \[\underset{\text{Ethylene glycol or Ethane -1, 2-diol}}{\mathop{HO-C{{H}_{2}}-C{{H}_{2}}-OH}}\,\] 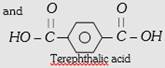 |
Copolymer, step growth linear condensation polymer | Fibre crease resistant, low moisture absorption, not damaged by pests like moths etc. | For wash and wear fabrics, tyre cords seat belts and sails. |
| (b) Glyptal or alkyd resin | \[\underset{\text{Ethylene glycol}}{\mathop{HO-C{{H}_{2}}-C{{H}_{2}}-OH}}\,\] and 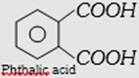 |
Copolymer, linear step growth condensation polymer | Thermoplastic, dissolves in suitable solvents and solutions on evaporation leaves a tough but not flexible film. | Paints and lacquers. | |
| (ii) Polyamides | |||||
| (a) Nylon-66 | \[\underset{\text{Adipic acid}}{\mathop{HO-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,{{[C{{H}_{2}}]}_{4}}\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-OH}}\,\] and \[\underset{\text{Hexamethyllenediamine}}{\mathop{{{H}_{2}}N-{{[C{{H}_{2}}]}_{6}}-N{{H}_{2}}}}\,\] | Copolymer, linear, step growth condensation polymer | Thermoplastic high tensile strength abrasion resistant. | Textile fabrics, bristles for brushes etc. | |
| (b) Nylon-610 | \[\underset{\text{Hexamethyllene}\,\,\text{diamine}}{\mathop{{{H}_{2}}N-{{[C{{H}_{2}}]}_{6}}-N{{H}_{2}}}}\,\] and \[\underset{\text{Sebacic acid}}{\mathop{HOOC{{[C{{H}_{2}}]}_{8}}COOH}}\,\] | Copolymer, linear, step growth | Thermoplastic, high tensile strength, abrasion resistant | (i) Textile fabrics, carpets, bristles for brushes etc. (ii) Substitute of metals in bearings. (iii) Gears elastic hosiery. | |
| (c) Nylon-6 or Perlon | 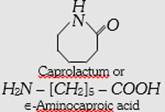 |
Homopolymer, linear | Thermoplastic high tensile strength abrasion resistant. | Mountaineering ropes, tyre cords, fabrics. | |
| (iii) Polyacryloni-trile or orlon or acrilon | PAN | \[C{{H}_{2}}=CH-CN\] | Copolymer | Hard, horney and high melting materials. | Orlon, arcrilon used for making clothes, carpets blankets and preparation of other polymers. |
You need to login to perform this action.
You will be redirected in
3 sec
